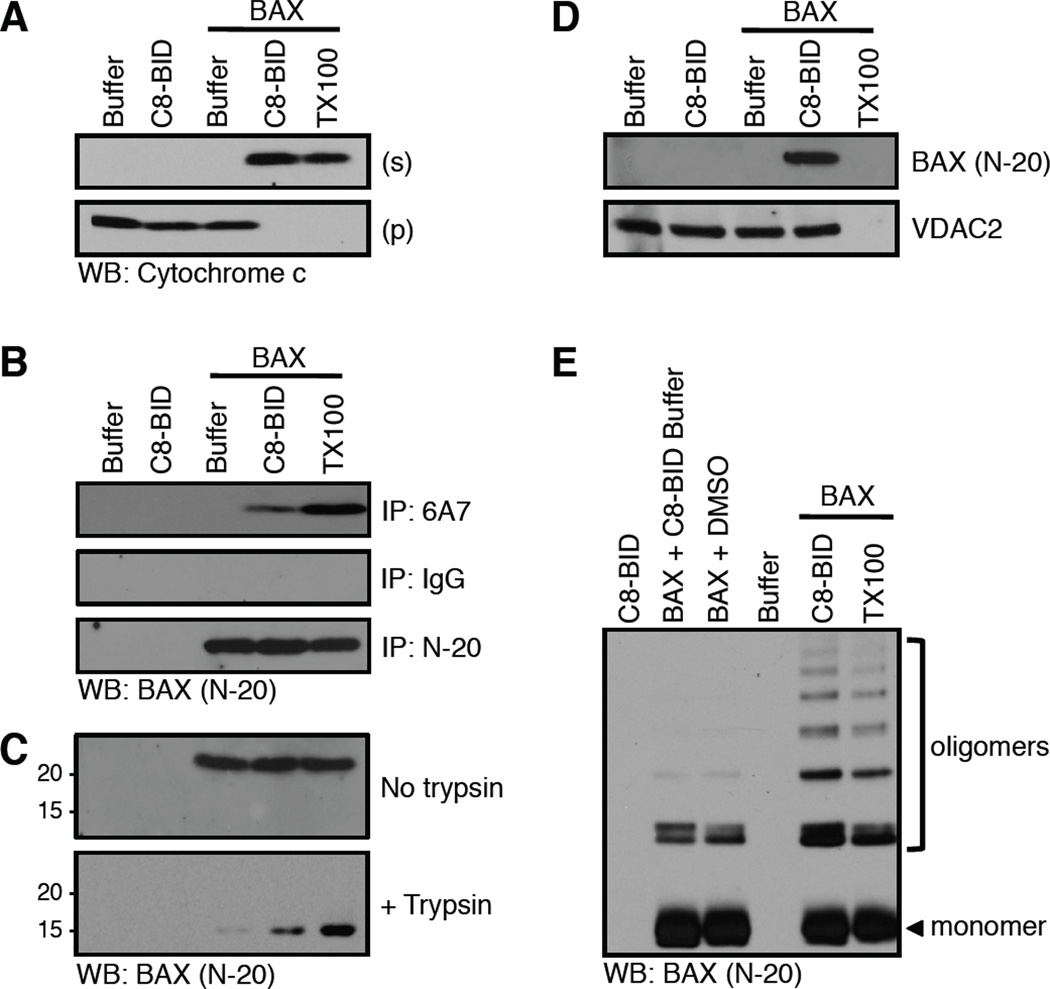
Figure 7.
Conformational changes correlated with BAX activation and cytochrome c release. (A) Mitochondria purified from bak−/−bax−/− liver were incubated with C8-BID (10 nM) and BAX (25 nM) for 60 minutes at 37°C before fractionation, SDS-PAGE, and western blot for cytochrome c. This MOMP reaction was set-up in quintuplicate, using 100 µl reaction volumes and 100 µg HM for analyses in A–E. 0.1% Triton X-100 (TX100) solubilizes mitochondria to release cytochrome c. (B) The amino terminal region of BAX is revealed by C8-BID, and captured following 6A7 immunoprecipitation (IP). IgG is an isotype control IP; N-20 is a non-conformation specific IP. TX100 artificially activates BAX and is a positive control. (C) BAX is trypsin (0.17 µg/µl) resistant when activated by C8-BID. TX100 artificially activates BAX and is a positive control. (D) C8-BID induced BAX integration into the OMM resists alkali extraction. VDAC2 is an integral OMM protein that is not carbonate sensitive. TX100 solubilizes mitochondria so both OMM integrated and associated proteins are not detectible as no pellet can be analyzed. (E) Cysteine cross-linking with BMH (1 µM) reveals BAX oligomerization induced by C8-BID. TX100 artificially activates BAX and is a positive control.