Figure 5.
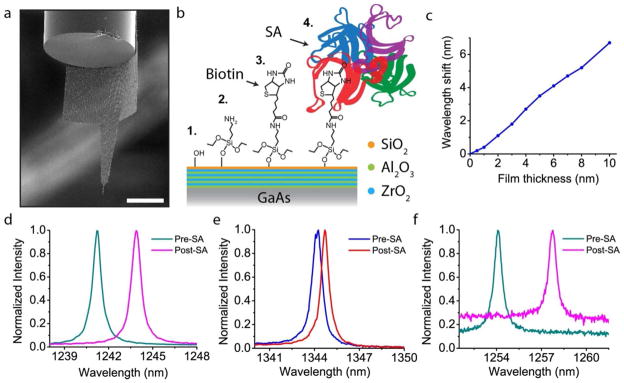
Nanoprobe detection of Streptavidin binding. a, SEM image of a modified nanoprobe that has extended ‘wings’ meant to wrap around the edge of the optical fiber, thus preventing sticking. b, Illustration of the surface chemistry for protein detection. The original, nanolaminate-coated GaAs, is coated with an additional layer of silica which has terminal hydroxyl groups. Aminosilanization with APTES yields an amine-terminated surface to which biotin binds. Finally Streptavidin specifically binds to the surface biotin molecules. c, Finite-difference frequency domain (FDFD) simulation of the expected wavelength shift as the organic film thickness is increased. The film was modeled as a uniform layer of refractive index equal to 1.45. d, Spectra of a chip-bound nanobeam both before and after SA adsorption, demonstrating a clear and large redshift of the cavity peak. e, Non-specific binding of a different beam cavity shows a much smaller redshift of 0.5 nm. f, Spectra of a nanoprobe device (shown in 5a) for the same specific binding chemistry. As in d, the redshift is clear and large. The difference in background PL is due to slightly different focus conditions of the laser spot, however, this has no bearing on the wavelength information. Scale bar, 50 μm.
