Fig. 4.
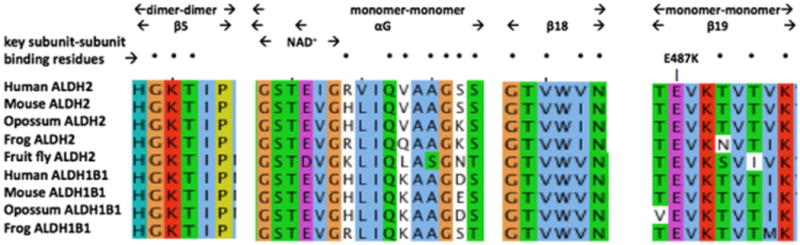
Comparative amino acid sequences alignments for ALDH2 and ALDH1B1 subunit binding domains. See Tables 1 and 2 for sources of ALDH1B1 and ALDH2 sequences, respectively. Three regions for vertebrate ALDH2 and ALDH1B1 sequences are shown, including dimer–dimer (β5); NAD+ binding site, monomer–monomer αG and β18, and monomer–monomer (β19). Amino acid residues are color coded: yellow for P (proline); green for hydrophilic amino acids, S (serine), Q (glutamine), N (asparagine), and T (threonine); brown for glycine (G); light blue for hydrophobic amino acids, L (leucine), I (isoleucine), V (valine), M (methionine), W (tryptophan); dark blue for amino acids, T (tyrosine) and H (histidine); purple for acidic amino acids, E (glutamate) and D (aspartate); and red for basic amino acids, K (lysine) and R (arginine). The site of the ALDH2*2 variant (E487K) is identified by the arrow.
