Fig. 3.
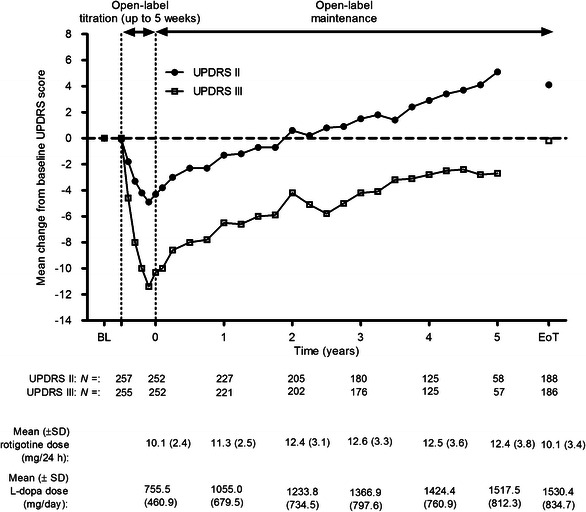
Change from baseline (visit two of double-blind study) UPDRS part II (ADL) and part III (motor function) scores during open-label treatment in SP715; safety set, observed cases. Mean rotigotine and l-dopa doses shown by timepoint. ADL activities of daily living, BL double-blind baseline, EoT end of treatment, l- dopa levodopa, SD standard deviation, UPDRS united Parkinson’s disease rating scale. Patients were followed for up to 4 years; data are not shown beyond 3.5 years due to <50 patients with measurements at these timepoints
