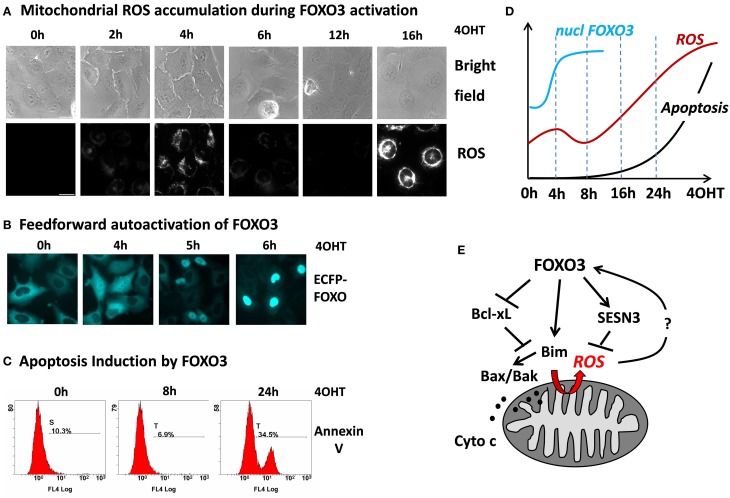
Figure 2.
FOXO3 activation causes biphasic ROS accumulation at mitochondria leading to feed forward activation and apoptosis. SH-EP neuroblastoma cells expressing a conditional FOXO3-ERtm protein were treated with 4-OH-tamoxifene (4OHT), which leads to functional activation of this transgene. FOXO3 induces a first ROS accumulation at 4 h with complete SESN3-dependent decay of ROS between 8 and 14 h and strong secondary accumulation of ROS afterwards that finally leads to apoptotic cell death. Accumulation of ROS was detected using MitoTracker Red CMH2XROS (Invitrogen) by live-cell imaging in an Axiovert200M microscope (Zeiss) (A). Subsequent to the first ROS peak triggered by FOXO3(A3)ERtm nuclear accumulation of ECFP-FOXO3wt is observed suggesting amplifying feed forward activation of additional FOXO3 molecules after primary FOXO3 activation (B). Apoptosis was determined by Annexin-V staining in a FC500 flow cytometer (Beckman-Coulter) (C). The chronology of cellular events in response to FOXO3 activation: after 4 h ECFP-tagged FOXO3 starts to accumulate in the nucleus (blue line, data based on quantification of nuclear FOXO3 by live cell imaging analysis), ROS accumulates first after 4 h, followed by decay to background ROS levels and a second much more intensive ROS accumulation (red line) that also marks the onset of cell death (black line, based on time course experiments using flow cytometric analysis of annexin-V staining). The data was compiled from analyses shown in (A–C), additional unpublished data or data from (Hagenbuchner et al., 2012a) (D). Model of cell death/stress resistance regulation by FOXO3 involving the biphasic accumulation of mitochondrial ROS downstream of Bim, which may constitute a feed forward signal to overcome the ROS-protective capacity of SESN3 (E).