Abstract
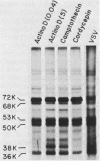
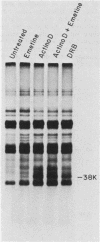
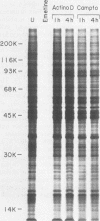
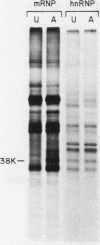
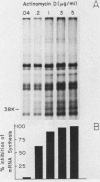
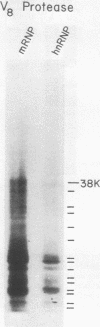
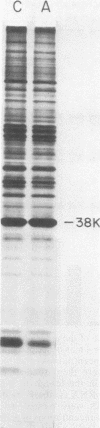
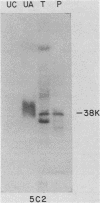
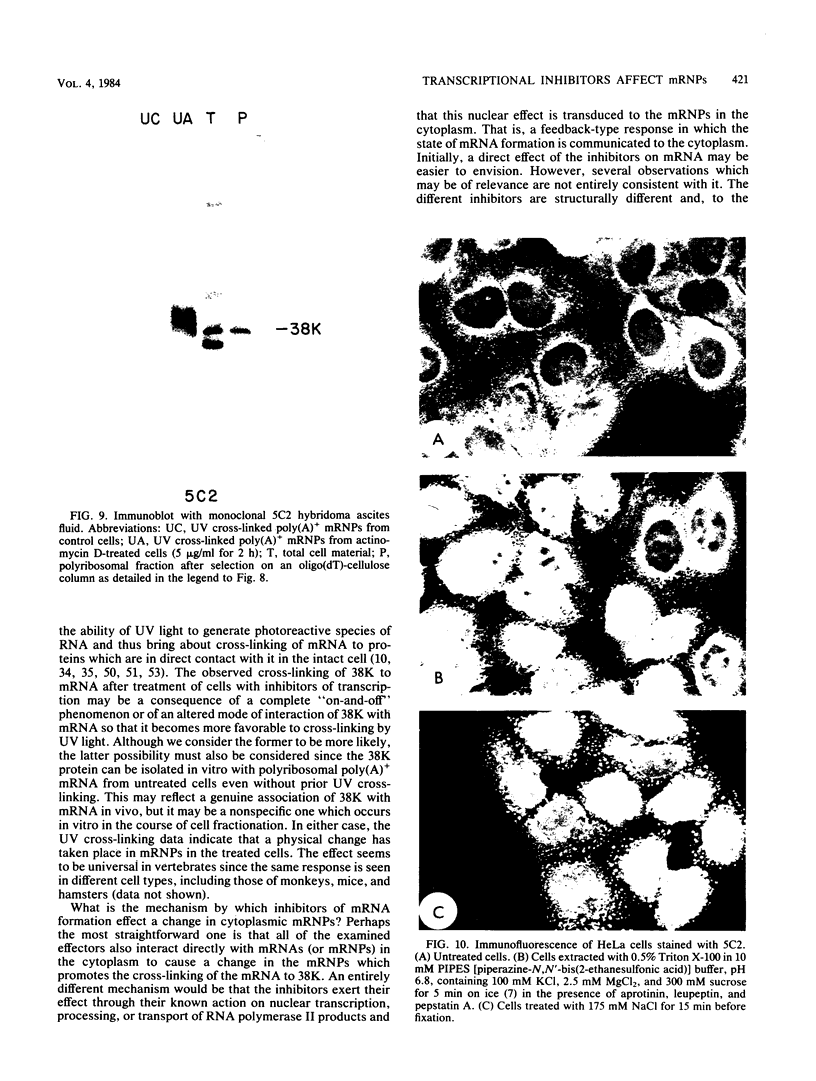
Exposure of intact cells to UV light brings about cross-linking of polyadenylated mRNA to a set of cytoplasmic proteins which are in direct contact with the mRNA in vivo. Substantial amounts of an additional protein of molecular weight 38,000 (38K) become cross-linked to the mRNA when cells are treated with inhibitors of mRNA synthesis (actinomycin D, camptothecin, and 5,6-dichloro-1-beta-D-ribofuranosyl benzimidazole) or after infection with vesicular stomatitis virus. Cordycepin, which inhibits polyadenylation but not mRNA synthesis, has no such effect. Inhibitors of protein synthesis and of rRNA synthesis are also without effect on 38K cross-linking to mRNA. The onset of the effect of inhibitors of mRNA synthesis on the UV cross-linkable interaction between mRNA and 38K is rapid and reaches a maximal level in less than 60 min, and it is completely and rapidly reversible. In cells treated with actinomycin D, the amount of 38K which becomes cross-linked to mRNA is proportional to the extent of inhibition of mRNA synthesis. The association of 38K with mRNA during transcriptional arrest does not require protein synthesis because simultaneous treatment with the protein synthesis inhibitor emetine does not interfere with it. The effectors which promote the interaction of 38K with mRNA do not affect the proteins which are in contact with polyadenylated heterogeneous nuclear RNA and do not markedly affect protein synthesis in the cell. The 38K protein can be isolated with the polyribosomal polyadenylated fraction from which it was purified, and monoclonal antibodies against it were prepared. Immunofluorescence microscopy shows mostly cytoplasmic and some nuclear staining. These observations demonstrate that commonly used inhibitors of transcription affect the physical state of messenger ribonucleoproteins in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S. Interaction of HeLa cell proteins with RNA. J Mol Biol. 1970 Feb 14;47(3):263–273. doi: 10.1016/0022-2836(70)90301-3. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butcher P. D., Arnstein H. R. Efficient translation and polyribosome binding of 125I-labelled rabbit globin messenger ribonucleoprotein. FEBS Lett. 1983 Mar 7;153(1):119–124. doi: 10.1016/0014-5793(83)80130-6. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Geoghegan T., Bergmann I., Brawerman G. Studies on the efficiency of translation and on the stability of actin messenger ribonucleic acid in mouse sarcoma ascites cells. Biochemistry. 1979 Jul 10;18(14):3153–3159. doi: 10.1021/bi00581a037. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
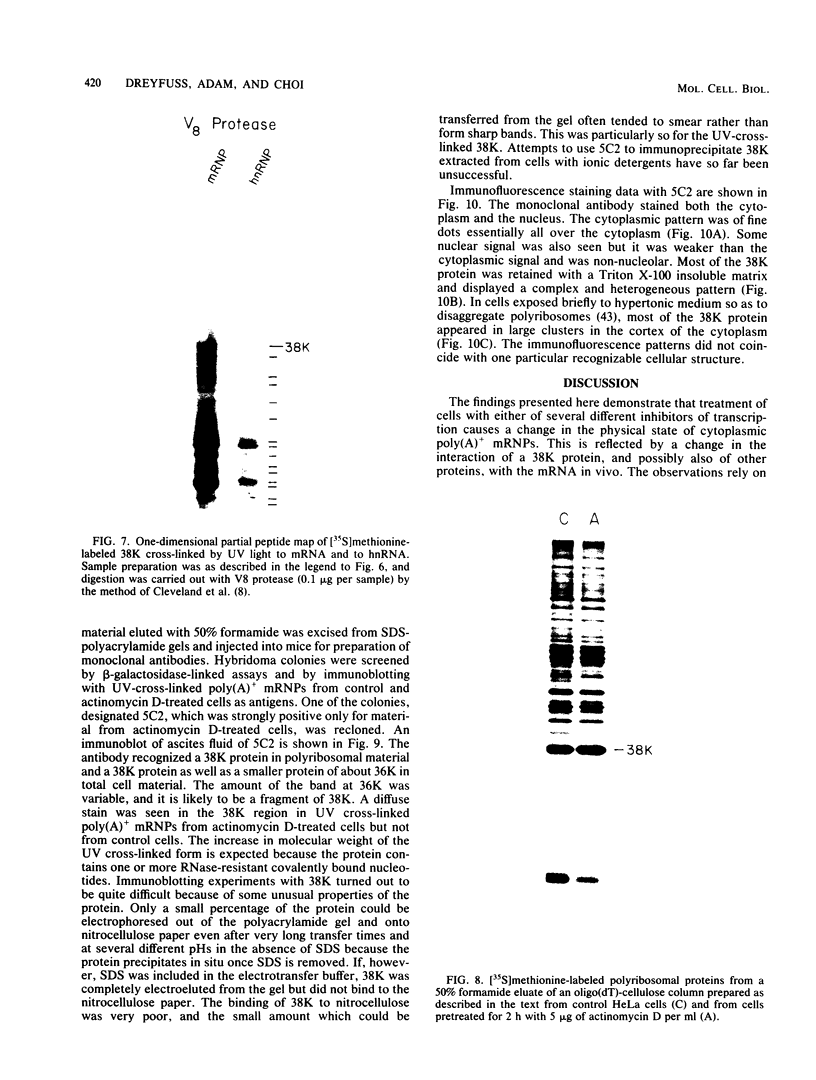
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Craig N. C. On the regulation of the synthesis of ribosomal proteins in L-cells. J Mol Biol. 1971 Jan 14;55(1):129–134. doi: 10.1016/0022-2836(71)90288-9. [DOI] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyházi E., Pigon A., Rydlander L. 5,6-Dichlororibofuranosylbenzimidazole inhibits the rate of transcription initiation in intact Chironomus cells. Eur J Biochem. 1982 Mar 1;122(3):445–451. doi: 10.1111/j.1432-1033.1982.tb06458.x. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E. DRB-induced premature termination of late adenovirus transcription. Nature. 1978 Apr 13;272(5654):590–593. doi: 10.1038/272590a0. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Girard M., Baltimore D. The effect of HeLa cell cytoplasm on the rate of sedimentation of RNA. Proc Natl Acad Sci U S A. 1966 Sep;56(3):999–1002. doi: 10.1073/pnas.56.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I. H., Friedman P. A. Antibiotics and nucleic acids. Annu Rev Biochem. 1971;40:775–810. doi: 10.1146/annurev.bi.40.070171.004015. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Proteins crosslinked to messenger RNA by irradiating polyribosomes with ultraviolet light. Nucleic Acids Res. 1980 Dec 11;8(23):5685–5701. doi: 10.1093/nar/8.23.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. The polyribosomal mRNA--protein complex is a dynamic structure. Proc Natl Acad Sci U S A. 1981 May;78(5):2923–2926. doi: 10.1073/pnas.78.5.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Rubin B. Y., Holmes S. L. Regulation of interferon action in human fibroblasts: transient induction of specific proteins and amplification of the antiviral response by antinomycin D. Virology. 1981 Jun;111(2):331–340. doi: 10.1016/0042-6822(81)90337-8. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Pluskal M. G., Sarkar S. Thermal chromatography of eukaryotic messenger ribonucleoprotein particles on oligo (dT)-cellulose. Evidence for common mRNA-associated proteins in various cell types. FEBS Lett. 1979 Jan 1;97(1):84–90. doi: 10.1016/0014-5793(79)80058-7. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R. Composition and properties of messenger ribonucleoprotein fragments containing and lacking polyadenylate. Biochim Biophys Acta. 1978 Nov 21;521(1):217–228. doi: 10.1016/0005-2787(78)90264-2. [DOI] [PubMed] [Google Scholar]
- Kessel D. Effects of camptothecin on RNA synthesis in leukemia L1210 cells. Biochim Biophys Acta. 1971 Aug 26;246(2):225–232. doi: 10.1016/0005-2787(71)90131-6. [DOI] [PubMed] [Google Scholar]
- Kessler-Icekson G., Singer R. H., Yaffe D. The capacity of polyadenylated RNA from myogenic cells treated with actinomycin D to direct protein synthesis in a cell-free system. Eur J Biochem. 1978 Aug 1;88(2):403–410. doi: 10.1111/j.1432-1033.1978.tb12462.x. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Poly (A)-rich ribonucleoprotein complexes from HeLa cell messenger RNA. J Biol Chem. 1976 Oct 10;251(19):5888–5894. [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz R. D. The effect of ethidium bromide on mitochondrial DNA synthesis and mitochondrial DNA structure in HeLa cells. J Cell Biol. 1971 Oct;51(1):116–122. doi: 10.1083/jcb.51.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand L., Ruddle F. H. Stimulation of in vitro translation of messenger RNA by actinomycin D and cordycepin. Science. 1977 Jul 22;197(4301):381–383. doi: 10.1126/science.17919. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Setyono B., Spindler E., Köhler K. Comparison of proteins bound to the different functional classes of messenger RNA. Biochim Biophys Acta. 1976 Apr 2;425(4):373–383. doi: 10.1016/0005-2787(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2208–2212. doi: 10.1073/pnas.78.4.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Mittleman B., Zandomeni R., Weinmann R. Mechanism of action of 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. II. A resistant human cell mutant with an altered transcriptional machinery. J Mol Biol. 1983 Apr 15;165(3):461–473. doi: 10.1016/s0022-2836(83)80213-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Schimke R. T. Regulation of protein synthesis in chick oviduct. 3. Mechanism of ovalbumin "superinduction" by actinomycin D. J Biol Chem. 1973 Mar 10;248(5):1502–1512. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- REVEL M., HIATT H. H. THE STABILITY OF LIVER MESSENGER RNA. Proc Natl Acad Sci U S A. 1964 May;51:810–818. doi: 10.1073/pnas.51.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E., Goldberg I. H. Actinomycin and nucleic acid function. Prog Nucleic Acid Res Mol Biol. 1964;3:183–234. doi: 10.1016/s0079-6603(08)60742-4. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Schwartz H., Darnell J. E. The association of protein with the polyadenylic acid of HeLa cell messenger RNA: evidence for a "transport" role of a 75,000 molecular weight polypeptide. J Mol Biol. 1976 Jul 15;104(4):833–851. doi: 10.1016/0022-2836(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Regulation of human interferon production. I. Superinduction by 5, 6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Virology. 1976 Apr;70(2):532–541. doi: 10.1016/0042-6822(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C., Sakore T. D., Nordman C. E. Stereochemistry of actinomycin--DNA binding. Nat New Biol. 1971 Jun 16;231(24):200–205. doi: 10.1038/newbio231200a0. [DOI] [PubMed] [Google Scholar]
- Tamm I., Sehgal P. B. Halobenzimidazole ribosides and RNA synthesis of cells and viruses. Adv Virus Res. 1978;22:187–258. doi: 10.1016/s0065-3527(08)60775-7. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Van Eekelen C. A., Mariman E. C., Reinders R. J., Van Venrooij W. J. Adenoviral heterogeneous nuclear RNA is associated with host cell proteins. Eur J Biochem. 1981 Oct;119(3):461–467. doi: 10.1111/j.1432-1033.1981.tb05630.x. [DOI] [PubMed] [Google Scholar]
- Vincent A., Goldenberg S., Scherrer K. Comparisons of proteins associated with duck-globin mRNA and its polyadenylated segment in polyribosomal and repressed free messenger ribonucleoprotein complexes. Eur J Biochem. 1981 Feb;114(2):179–193. doi: 10.1111/j.1432-1033.1981.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J Mol Biol. 1970 Apr 28;49(2):319–342. doi: 10.1016/0022-2836(70)90248-2. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Kumar A., Warner J. R. Ribosome formation is blocked by camptothecin, a reversible inhibitor of RNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3009–3014. doi: 10.1073/pnas.68.12.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]