Abstract
In addition to natural stressors, populations are increasingly exposed to chemical pollutants released into the environment. We experimentally demonstrate the loss of resilience for Daphnia magna populations that are exposed to a combination of natural and chemical stressors even though effects on population size of a single stressor were cryptic, i.e. hard to detect statistically. Data on Daphnia population demography and along with model-based exploration of our predator-prey system revealed that direct trophic interactions changed the population size-structure and thereby increased population vulnerability to the toxicant which acts in a size selective manner. Moreover, population vulnerability to the toxicant increases with predator size and predation intensity whereas indirect trait-mediated interactions via predator kairomones may buffer chemical effects to a certain extent. Our study demonstrates that population size can be a poor endpoint for risk assessments of chemicals and that ignoring disturbance interactions can lead to severe underestimation of extinction risk.
Risk assessment of chemicals is strongly regulated in Europe and North America. For pesticides and other toxicants, risk is quantified via the ratio of a predicted environmental exposure concentration and a concentration causing a toxic effect (Europe) or its inverse (North America). Lethal and sublethal toxic effects, however, are determined in standard laboratory tests, with individual organisms. What these individual-level effects mean at the level of populations, communities, and ecosystems remains an open question. To take this uncertainty into account, safety factors are used in the estimate of concentrations that unlikely result in environmental effects, but their choice is arbitrary1. So far, safety factors seemed to be conservative enough to prevent Rachel Carson's Silent Spring2 but concern about the lack of ecological realism in current risk assessment schemes is increasing, since the number and intensity of stressors on natural populations have reached unprecedented levels. In particular, it remains unclear which endpoints or metrics for ecological risk assessment should be considered at the population level. Average abundance, intrinsic rate of increase, or recovery time have been proposed but do they really relate to risk in an ecologically meaningful way?
In ecology, focus has shifted over the last two decades from equilibria to negative feedback, transient dynamics3 and resilience, defined as the ability to maintain internal relationships and functioning despite disturbances and stress4. Resilience is the result of buffer mechanisms, which only have a certain capacity5. Stress beyond this capacity leads to sudden changes or regime shifts, which for populations may correspond to extinction. Regime shifts have been observed for ecosystems such as savannas and coral reefs but might also be an issue in species conservation, the most prominent example being the decline in honeybees and amphibians6,7. So far, no satisfactory monocausal explanation of their decline has been found. Rather, multiple stressors might have caused the loss of their resilience.
For chemical stress, several studies have demonstrated that a combination of toxicants and species interactions can impose much higher risk on populations than the toxicant alone. Community processes that can alter the magnitude of toxicant effects include interspecific competition8, parasitism9,10, presence of predator kairomones11,12 and predation13,14. These processes affect not only abundance but also intraspecific competition and population size-structure. Since individuals' susceptibility might differ across life stages, body sizes, population size-structure and the timing of exposure, there will be consequences for the susceptibility of populations to toxicants15,16.
Here we raised the question of whether using population abundance as an endpoint for ecological risk assessment is sufficient to indicate the risk of losing resilience when populations are exposed to a combination of chemical stress and predator effects. As a model system we used the water flea Daphnia magna, a standard test species for ecotoxicology. It has previously been shown for the case of Daphnia that predator conditioned culture medium or the presence of predators altered egg size17, resulted in a higher age at maturity18 and in smaller clutch sizes11. However, rapid juvenile development and high reproductive output were also observed19. Previous laboratory studies applying artificial predation by manually removing fixed portions of prey populations revealed alterations in prey abundance14 and species composition20 as well as changes in recovery times after insecticide pulse exposure21.
Predation may interact with toxicant effects via size-selectivity of both stressors. Predators tend to specialize in particular sizes or developmental stages in their choice of prey types22,23. Such size selectivity was frequently found in fish24,25 and invertebrate predators26,27,28. The outcome of predation has also been shown to vary with predator size29,30,31. Changes in size selectivity and functional response during life history were reported in insect predators of the genus Notonecta28,32,33. Within the five larval stages of Notonecta, early instars usually prefer small crustaceans, whereas later instars forage on larger prey34.
In this study, we investigated the interaction of predation and toxic stress using an integrative approach of laboratory experiments and independent mechanistic model simulations. In our laboratory experiments, we exposed Daphnia populations to pulses of a toxicant, to continuous predation by Notonecta alone, and to a combination of both. To capture size-selective effects, not only abundance but also size-structure was recorded. To explore scenarios and hypotheses beyond the constraints of our experiment, an individual-based model of our system was used to test effects of predator size, predation intensity and the significance of certain, isolated mechanisms on the Daphnia population's resilience.
Results
Experimental results
Population size
Size of Daphnia magna populations increased during the first four weeks of the experiment. After reaching maximum abundance, the total number of daphnids decreased and control populations leveled off after reaching environmental capacity at a mean density of 113.4 ± 31.49 (mean ± standard deviation) individuals per L (Figure 1, details given in Appendix S3 in Supporting Information). The density of D. magna under predation did not significantly differ from control with a single exception 9 days after introduction of backswimmers (Figure 1, P = 0.036). Thereafter, between day 14 and day 34 of the experiment (hereafter referred to as the recovery phase), total abundances of Daphnia slightly increased compared to control, with differences not being significant. The first p-353 nonylphenol pulse exposure resulted in a significant short-term reduction in D. magna population size as compared to control (P = 0.036), whereas the second nonylphenol pulse did not have a significant effect on the total daphnid abundance (P = 0.158). In the systems exposed to both predator and nonylphenol, population density did not significantly differ from single stressors until day 40 of the experiment (P = 0.065), including the first pulse exposure. Following the second pulse exposure the total number of Daphnia was significantly reduced compared to predation and nonylphenol treatments (P = 0.022). Finally, one out of three Daphnia populations exposed to the combined stressor went extinct and the other two populations were dramatically reduced in size (Figure 1); only in one of the three replicates did the population abundance start to increase again on the last day of the experiment.
Figure 1. Total number of Daphnia magna during the course of the experiment.
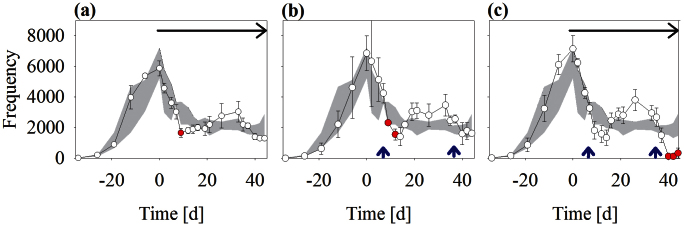
Daphnia populations were exposed to three different disturbance scenarios: (a) Notonecta maculata predation, (b) pulse exposures of p353-nonylphenol, and (c) combined disturbance by means of nonylphenol and predation. The black arrow marks the period of predator exposure, blue arrows indicate nonylphenol two-day pulse exposure periods. Dots represent mean and standard deviations of three replicated measurements. The grey shade symbolizes the range of control populations (range between minimum and maximum control abundance) and red dots indicate significant difference from control in ANOVA with subsequent Tukey post-hoc test.
Population size-structure
Comparison between first (day 7) and second (day 35) nonylphenol pulse exposures revealed that size-structure of control populations had changed during the course of the experiment (Figure 2), in particular, the number of adults (> 2.6 mm) steadily increased with time. Although backswimmer predation did not greatly affect total abundances, we observed alterations in D. magna population size-structure. The comparison of size distribution between control and disturbed populations (Figure 2) suggests that small sized D. magna were predominantly reduced by the predation of 1st instar backswimmers. Abundance of juvenile daphnids (> 1.3 mm & ≤ 2.6 mm) was significantly lower in predation systems as compared to control nine days after backswimmer introduction (P = 0.01). By contrast, in the presence of older backswimmers, numbers of adult D. magna were significantly reduced at the end of the recovery phase (from day 33, P < 0.001, Figure 3). The lack of significant differences in total abundance between predation and control can thus be attributed to the significantly higher numbers of neonate D. magna from day 37.
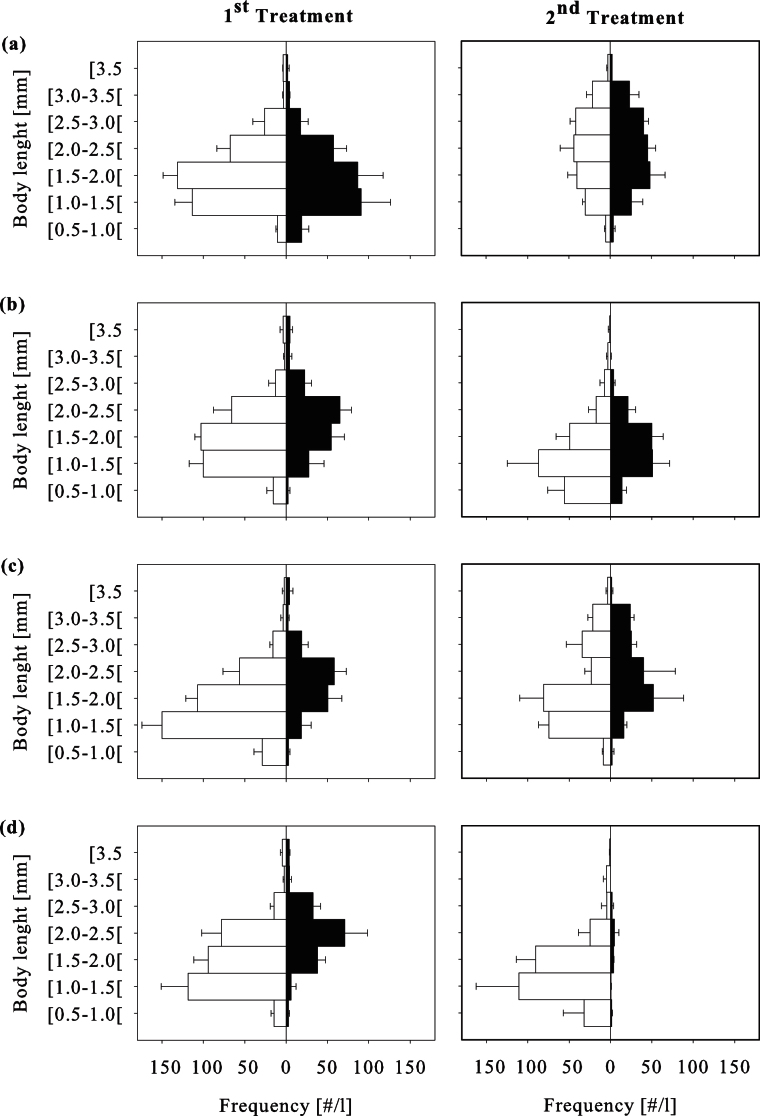
Figure 2. Population demography of Daphnia magna based on body length measured before (white bars) and after (black bars) the first (left) and second (right) p353-nonylphenol pulse exposure period; bars represent mean and standard deviation of three replicates in a control (a) and three different treatments: (b) predation by Notonecta maculata juveniles, (c) exposure to nonylpenol, and (d) a combination of both nonylphenol exposure and predation.
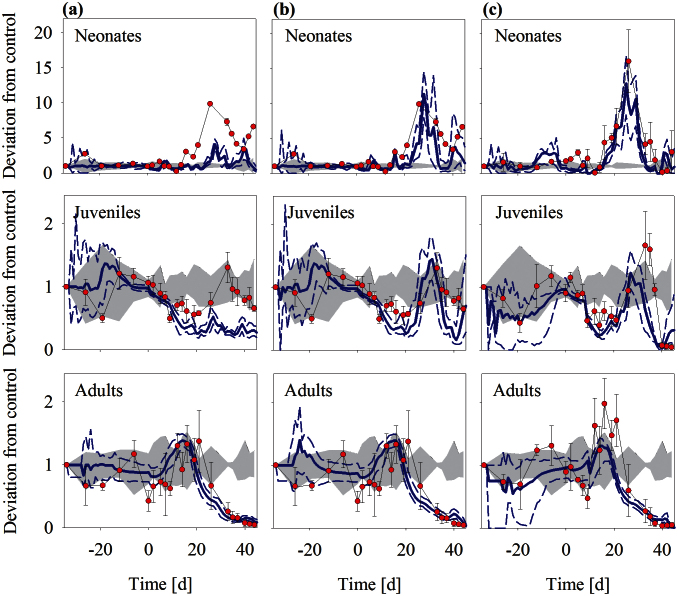
Figure 3. Importance of life-history plasticity in Daphnia magna population response to predation (a and b) and both predation and chemical stress (c).
Dynamics are shown separated by D. magna size classes within the populations: neonates (≤ 1.3 mm), juveniles (> 1.3–2.6 mm) and adult (> 2.6 mm). Simulated (blue lines) and measured population (red dots, mean ± standard deviation, n = 3) dynamics are presented in relative deviation to mean control abundance, with measured control range (range between minimum and maximum control abundance relative to mean) represented by grey shades. Dotted lines indicate minimum and maximum and solid line represents mean of 100 Monte-Carlo simulations. Panel a shows simulated population response to N. maculata predation whereas in panels b and c additional effects of predator kairomones were simulated. Data points in a and b are identical.
In single stressor settings, D. magna neonates were most severely affected by nonylphenol treatments (Figure 2). During the first exposure period, neonates were reduced in numbers by a factor of 12.3 and a significant difference from control was apparent three days after termination of the nonylphenol treatment (P = 0.002). During the recovery phase, neonate abundance increased and significantly exceeded control level (P = 0.014). The second nonylphenol treatment reduced the number of neonate D. magna by a factor of 5.7 whereas the difference to control treatment was, however, not significant. Abundances of juvenile and adult daphnids were significantly reduced during the first nonylphenol exposure period by a factor of ~ 2 (P = 0.01, P = 0.028 respectively). Thereafter, the number of both juveniles and adults did not significantly differ from control, with the exception of the last day of the experiment where a significant reduction in adult abundance was apparent (P < 0.001).
In combined stressor settings, the number of neonate D. magna was reduced by a factor of 13.9 as a result of the first nonylphenol pulse exposure. The size distributions of D. magna populations indicate that the effect of the first nonylphenol treatment was more pronounced in combined stressor systems compared to single stressors (Figure 2). However, abundances of the three size classes did not significantly differ from single stressor scenarios (P = 0.06). At the initiation of the second nonylphenol pulse exposure populations exposed to combined stressors largely consisted of small bodied daphnid specimens whereas adult Daphnia accounted for only 4.1% of the population. In the following days, neonate and juvenile abundance was significantly reduced (P < 0.001 and P = 0.037 respectively) by factors of 36.6 and 27.1 respectively as compared to pre-exposure conditions. In the end, adult D. magna were totally absent from two of the three combined stressor systems.
Toxicant concentrations and food conditions
Measured nonylphenol concentrations were on average 23.1% below the nominal concentration of 0.45 μM considered for the experiment (Table 1). Density of algae that were not consumed decreased during the time course and reached a plateau on day -6 (Table 2). No significant difference was found in the density of algae between control and treatment systems (Table 2), with the exception of day -12 (P = 0.009) indicating that populations equally reached carrying capacity of the systems following the peak density.
Table 1. Mean and standard deviation of measured nonylphenol concentration in test media (n = 3) during the first (days 7 and 8) and second (days 35 and 36) 2-day pulse exposure period. NP: p353-nonylphenol exposure; Np-Nm: combined stressor.
| Treatment | p353-nonylphenol concentrations [μM] | |||
|---|---|---|---|---|
| Day 7 | Day 8 | Day 35 | Day 36 | |
| NP | 0.47 ± 0.163 | 0.24 ± 0.088 | 0.38 ± 0.192 | 0.16 ± 0.070 |
| NP-Nm | 0.37 ± 0.122 | 0.18 ± 0.028 | 0.18 ± 0.136 | 0.10 ± 0.26 |
Table 2. Mean and standard deviation of food availability (n = 3) given as total organic carbon [mg/L], letters indicate differences in one way Anova with subsequent Tukey HSD post-hoc test. C: control, Nm: Notonecta maculata predation, NP: p353-nonylphenol exposure; Np-Nm: combined stressor.
| Day | C | Nm | NP | NP-Nm | p |
|---|---|---|---|---|---|
| −26 | 4 ± 0.29A | 4.6 ± 0.98A | 4.3 ± 1.36A | 4.7 ± 1.55A | 0.120 |
| −19 | 3.8 ± 1.55A | 6.6 ± 1.08A | 5.6 ± 0.42A | 6.1 ± 3.63A | 0.956 |
| −12 | 1.3 ± 0.2A | 2.6 ± 0.89B | 3.3 ± 1.07B | 4 ± 0.38B | 0.009 |
| −6 | 1.2 ± 0.36A | 1 ± 0.15A | 1.7 ± 0.06A | 1.3 ± 0.23A | 0.105 |
| 5 | 1.3 ± 0.71A | 1.4 ± 0.64A | 1.7 ± 0.26A | 2.1 ± 0.11A | 0.288 |
| 16 | 1 ± 0.17A | 1.5 ± 0.23A | 1.1 ± 0.21A | 1.2 ± 0.23A | 0.221 |
| 26 | 1.7 ± 0.11A | 1.2 ± 0.41A | 1.6 ± 0.15A | 1.2 ± 0.25A | 0.135 |
| 36 | 1.4 ± 0.47A | 1.4 ± 0.26A | 1.4 ± 0.64A | 1.5 ± 0.52A | 0.476 |
Modeling results
The individual-based population model was run with and without consideration of predator kairomone effects on Daphnia life history. Without kairomone effects the impact of the predator was due to feeding only whereas in kairomone simulations D. magna adults produced more but smaller neonates. The importance of life-history plasticity in D. magna becomes apparent when comparing neonate and juvenile dynamics predicted by the two models (Figures 3A and 3B). Without consideration of kairomone effects, the model predicts only a slight increase in neonate abundance during the recovery phase of the experiments between days 15 and 30, largely as a consequence of increasing food availability. However, in order to capture the full magnitude of reproductive output and the subsequent increase in juvenile abundance, life-history plasticity needed to be included in the model. Consequently, with consideration of kairomone effects, the model is able to capture the dynamics of population size-structure reasonably well.
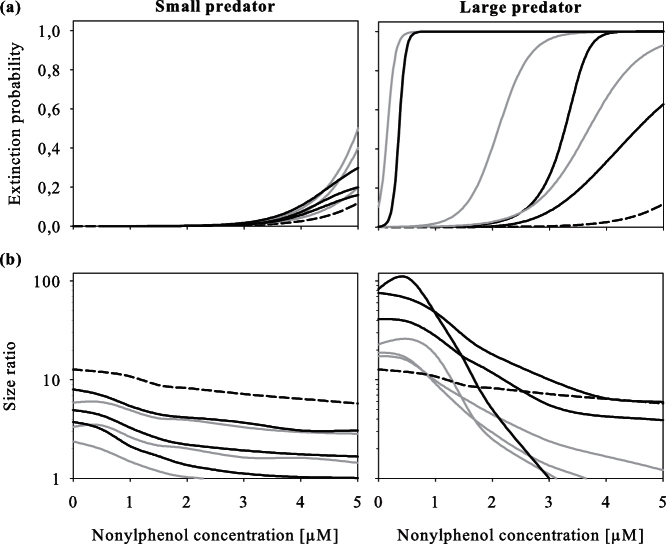
To examine the effect of predator size, predation intensity and the presence of kairomones on Daphnia population size-structure and on population vulnerability to the toxicant, we simulated larger environments that were able to carry more than a single backswimmer. Simulated extinction probabilities revealed that vulnerability of D. magna populations to chemical stress increases with predator size and predation intensity whereas the presence of kairomones buffered chemical effects to a certain extent (Figure 4). The patterns in the D. magna body size ratio (≤ 2.6 : > 2.6 mm) as function of nonylphenol concentrations differed remarkably between the two predator sizes tested (Figure 4). As a general trend, size ratios decreased with increasing chemical concentration, i.e. a shift towards older and thus larger animals. Simulated kairomone effects generally led to a shift towards small D. magna as a consequence of higher reproductive outputs. In small predator simulations, the stepwise reduction of the size ratio with increasing predation intensity can be ascribed to the increasing predation pressure on small D. magna. For large predators, the preference for large daphnid prey led to more complex size ratio patterns: under low nonylphenol exposure concentrations, size ratio increased with increasing predation pressure because small Daphnia could emerge and grow due to reduced intraspecific competition for algae. In contrast, under high exposure a large fraction of small Daphnia was killed leading to an inverse size ratio trend. These effects were even more pronounced under kairomone effects. Higher predation pressure of the large N. maculata instar led to rapid changes in size-structure and extinction probability, irrespective of life-history plasticity in D. magna. The modelling results support the experimental findings that selective predation of large backswimmer instars promoted size classes that were more vulnerable to the toxicant leading to higher vulnerability in populations exposed to a combination of both chemical and natural stressor.
Figure 4. Extinction probability (a) and size ratio (b) in Daphnia magna populations as functions of nonlyphenol concentration simulated without (grey solid lines) and with (black solid lines) consideration of kairomone effects.
Endpoints were calculated for small (1st instar, left panel) and large (5th instar, right panel) Notonecta maculata. Increasing line weight corresponds to increasing predation intensities (1–3 N. maculata specimens per system), with dashed lines representing simulations without predation. Extinction probabilities were recorded 10 days after initializing a 2-day pulse exposure and effects on population size-structure (mean of 100 Monte-Carlo simulations) 3 days after initializing exposure.
Discussion
Population dynamics of the cladoceran filter feeder Daphnia magna were observed and simulated for different disturbance scenarios including pulse exposure to p353-nonylphenol, predation of the insect predator Notonecta maculata, and a combination of both. Effects of the single stressors on total abundance were almost entirely cryptic, i.e. hardly detectable. In contrast, the combination of predation by large backswimmers and the second nonylphenol pulse exposure drove the daphnid populations to the brink of extinction (Figure 1). In the following section we will provide a mechanistic explanation of the interactive effects observed. Furthermore, we will discuss what we have learned about buffer mechanisms and cryptic effects, followed by a discussion of the relevance of our findings for ecological risk assessments of chemicals.
Total abundance of Daphnia magna was only marginally affected by the predation of backswimmers. Similarly, laboratory populations of Daphnia pulex were able to persist when exposed to groups of Notonecta hoffmani with abundances not being significantly different to control systems35. The authors suggested that an increase of reproductive output and juvenile survival probably due to a release form starvation enables prey populations to maintain densities and compensate for predation. Obviously, the loss of individuals due to predation was buffered by changes in the internal organization and size-structure of Daphnia populations.
Using a realistic and mechanistic population model, we have shown that altered food conditions in combination with effects of life-history plasticity due to predator kairomones, led to increased reproduction and smaller offspring size (Figure 3). Only this combination of responses to predator effects could explain why effects on population dynamics, i.e. abundance, were cryptic while population size-structure changed dramatically.
Similar to fish, Notonecta species are usually considered as gape-limited predators preferring larger prey20. However, small backswimmer instars prefer small over larger prey28. Accordingly, early in the experiments differences in population size-structure in control populations can partly be ascribed to size-selective predation in N. maculata juveniles32. This resulted in a reduction of neonate and juvenile D. magna, although the impact of predation was limited due to lower feeding rates in small instars36. Later in our experiment, larger backswimmers with more intense predation and a preference for larger prey reduced the abundance of adult D. magna to extinction and thereby induced a significant shift in the size-structure of populations towards smaller daphnids.
Our model revealed that kairomone effects were important to fully capture the observed response of the Daphnia population to predators (Figure 3). When exposed to Notonecta kairomones daphnids tend to produce larger clutch sizes with individual neonates being smaller, thereby influencing offspring vulnerability to the chemical stressor. These effects are an example of non-trophic, trait-mediated interactions. Their relevance for community dynamics and structure is increasingly noticed37, a prominent example being ungulates in the Yellowtone National Park who forage in vegetation with better cover but less nutritional quality since wolves have been re-introduced38. Our study demonstrates that such effects also have to be taken into account if the ecological realism of risk assessment of chemicals is to be improved.
Another important mechanism causing alterations in the size-structure of D. magna populations in response to stress was intra-specific competition. A large number of neonate D. magna were produced when total abundance was below the carrying capacity of the experimental systems, i.e. during the initial phase of the experiment and after disturbances. As soon as the carrying capacity was reached, intra-specific competition for food and space presumably caused a reduction in reproduction, growth and survival. Reductions in the reproductive output and survival at individual level have previously been reported for D. magna as a consequence of food shortage39,40,41 and crowding41,42.
The toxicant used in our experiments, p353-nonylphenol, has previously been found to act in a size-dependent manner, with juvenile and adult D. magna being less sensitive than neonates43. Consequently, in accordance with the study of Agatz and co-workers44, we found that populations of predominantly small daphnids have been affected by lower nonylphenol pulse exposure. However, in our study, the response of the Daphnia populations to toxic stress differed with timing of exposure. A significant effect was observed at the first peak, whereas the second peak showed no significant effect on abundance. During the initial phase of the experiment small Daphnia were numerically dominant; this is typical for growing populations45. Therefore, the first nonylphenol pulse exposure affected a significant proportion of the population. Then, in the non-predated systems the proportion of larger D. magna steadily increased over time. Accordingly, the proportion of small-sized Daphnia that might be mostly affected by the toxicant was lower during the second exposure period and thus toxic stress did not result in a significant reduction in population size. With predation on the other hand, first small backswimmers reduced the number of small daphnids so that only a small proportion of the Daphnia populations were affected by the toxicant at the first peak. Consequently, the first nonylphenol exposure did not result in a significant difference between the predated systems and the ones exposed to the combined stressor.
Later in the experiment, predation of large backswimmers foraging on larger daphnids and the high reproductive output induced by reduced intraspecific competition led to a shift in population size-structure towards small Daphnia at the time of the second toxicant exposure. With small daphnids being more sensitive, a large proportion of the population was affected by the toxicant and finally became extinct or was driven to the brink of extinction.
In contrast, both intraspecific competition for food, leading to the well-known cohort-dominance suppression cycles46, and episodic addition of food are unlikely to explain our observations per se, as they are represented in the simulation model. However, spatial effects such as swarming and vertical migration might have effects. We observed that migration to the bottom of the aquarium was less pronounced in the absence of predators. Since our simulation model is non-spatial, it does not account for such effects, which might explain why population dynamics and size-structure predicted by the model are, although capturing the main trends and levels, not 100% perfect.
The above findings have implications for the analysis of resilience and multiple stressor scenarios. In ecology, resilience has become a central concept. It includes the notion of buffering disturbances to a certain limited capacity which can be exhausted by too strong or too long-lasting a stress. The reason for the limited capacity is that stressors have to cause some change in the system, otherwise they would not be considered stressors. In ecotoxicology, multiple stressors are customarily discussed in close connection with synergism. Synergistic effects are claimed whenever a combination of stressors amplifies effects, usually observed on the level of the individual organism, beyond additivity47. In our study, the combination of a natural and chemical stressor did not increase the effects on the organismal level but led to a nonlinear combination of effects at the population level. The effect of predation on prey individuals was buffered by reproduction at population level, subsequently leading to a shift in population size-structure (Figures 2 and 3). The effect of a second stressor finally led to the loss of resilience.
If resilience is lost then abrupt regime shifts occur, leading to a system that is organized in a fundamentally different way48. Our study is one of the first which, for a population under multiple stresses, combines experiments and modeling to provide mechanistic explanation of resilience and the loss of its buffering capacity. The lesson learned from our study is that in general the response of a population or ecological system to single stressors inevitably causes changes in structure and internal organization. These responses have different profiles, each changing the susceptibility to other stressors in a particular way. Assessing risk of single stressors might thus severely underestimate real risk. Ecologically relevant risk assessment requires a more comprehensive view including population structure and internal organization.
Traditionally, population ecology has a strong focus on abundance and it can be difficult enough to estimate abundance in real populations. However, in theoretical ecology, structured models49 and individual-based models50 are increasingly used to take into account differences among individuals and their adaptive behavior, including plasticity in physiology and life history. Population size is a summary statistic that ignores information about population structure and the state and behavior of individuals that in many, if not most, cases is vital to understand and predict the response of populations to changing conditions, including stress.
Nevertheless, population level endpoints (i.e., metrics for risk assessment) currently discussed in ecological risk assessment of chemicals still focus on abundance itself or abundance-related metrics: population growth rate51 or recovery of abundance to pre-disturbance levels52. These endpoints certainly contain important information, but our study has demonstrated that endpoints based on abundance might not detect real risk if, as in the current practice in risk assessment of chemicals, only single stressors and single populations are considered. As demonstrated in our study, effects of a toxicant can go undetected when ignoring population structure but become relevant when combining chemical stress with species interactions, both direct and trait-mediated.
For populations, demographic studies that include estimates of population vulnerability resulting from size-dependent differences in susceptibilities to toxicants offer a starting point in overcoming the limitation of traditional toxicity assessments based on individual organisms53. For communities, a multitude of factors and interactions such as competition, predation or parasitism complicate the empirical assessment of toxicant effects. Even if one or more of these interactions are addressed, results may depend on experimental settings, as shown here for the case of predation (Figure 4). The strong interaction observed in our experimental system appears to be largely due to strong size-dependent effects, in particular due to the relative sizes of predators and prey. In a natural system one might expect a more heterogeneous distribution of predator sizes and size-selectivity which need further investigation. We thus conclude that mechanistic and well-tested population and community models are needed to fully understand mechanisms behind the interaction of natural and chemical stressors. Routinely using experiments and models together, as in the present study, will ultimately help to develop schemes for a more realistic ecological assessment of chemicals.
Methods
Experimental study
In a laboratory experiment, we observed Daphnia magna population dynamics under pulsed stress of 4-(3,5 dimethyl-3 heptyl)phenol (p-353 nonylphenol), under predation by the backswimmer Notonecta maculata and with a combination of both, toxic stress and predation.
Test Animals
The experiment was carried out using D. magna clone 5. Daphnids were reared as described by Siehoff and colleagues54. Adults of N. maculata were originally collected from an outdoor mesocosm site and were cultured under laboratory conditions. Backswimmer juveniles were reared individually and fed ad libitum with daphnids. For culturing and experiments, animals were kept in a constant room set at 20 ± 1°C and a 16 h:8 h (light:dark) photoperiod. Artificial freshwater M4 was used as a medium55.
Experimental setup
The D. magna population experiment was conducted under semi-static conditions using 20 L glass aquaria. Replicate populations (n = 3) were initiated on day -34 with four 21–28 day old D. magna specimens as well as 10 third brood neonates that were < 24 h old. The development of populations was followed during a period of 78 days. Daphnids were fed with the green algae D. subspicatus in a concentration of 6 mg TOC day−1 population−1 until day -25, 15 mg between day -24 and day -11 and 12 mg from day -10. Food was provided daily on working days with the amount of algae tripled on Fridays. In all aquaria dead daphnids, carcasses, aborted or ephippial eggs and deposed algae were removed weekly using a suction hose and half of the medium was renewed.
N. maculata specimens (< 24 h old) were placed in prepared aquaria at Daphnia peak abundance on day 0 of the experiment; they grew from first to fifth instar during the remaining period of the test. Backswimmers were checked daily and dead ones were replaced by individuals of the same age. Two two-day nonylphenol pulse exposures were initiated on day 7 and day 35 respectively. For nonylphenol treatment a nominal concentration of 0.45 μM was chosen, which was below the EC50 value in D. magna acute 48 h-toxicity test using neonates43.
Sampling procedure
Control and treatment populations of D. magna were sampled weekly at the beginning of the experiments and up to three times per week after the introduction of backswimmers. For the quantification of D. magna population size and size-structure, a sample of 2 L was obtained from each aquarium by taking 28–30 subsamples in a predefined pattern. For sampling, a plastic tube (30 × 2 cm) was vertically plunged into the water column. The upper end of the tube was sealed, but a small opening allowed air to leave the cylinder. Removal of enclosed Daphnia and water from the aquaria was possible when closing the upper opening. Daphnia samples were sieved (300 μm mesh), transferred to petri dishes and were returned to the aquaria after scanning. Daphnids were counted and body lengths of individuals (excluding spine) were measured digitally.
Density of algae cells was measured photometrically (Hitachi 100–40, 720 nm) on eight occasions during the course of the experiment. Therefore, a 50 ml sample of test medium was taken from each aquarium prior to feeding with algae. Total organic carbon was estimated from the empirical regression TOC = 269.73 A/(2.76 - A) with A being the measured absorbance.
Nonylphenol exposure
P-353 nonylphenol was synthesized as described previously56, yielding a chemical purity of > 97%. Acetone (Roth, purity 99.9%, Rotisolv-HPLC) was used as a carrier solvent. Aliquots of the nonylphenol stock solution (27.3 μM) were added to 200 ml medium and stirred before treating full aquaria. Nonylphenol exposures were terminated by fully replacing media after carefully cleaning aquaria with ethanol and water. Controls and predation systems were treated in the same way. Exposure concentrations were verified as described in Appendix S1 in Supporting Information.
Data analysis
For statistical analysis daphnids were graded into three size classes; these were neonates (≤ 1.3 mm), juveniles (> 1.3 & ≤ 2.6 mm) and adults (> 2.6 mm). Differences in D. magna total abundance and abundances within daphnid size classes between control and disturbance scenarios were tested by means of a one-way analysis of variance (Anova) with subsequent Tukey-HSD posthoc analysis, since most data passed Levene's test of homoscedasticity and Shapiro-Wilk test of normal distribution. Statistical analyses were conducted using PASW Statistics 18 (SPSS Inc., 2009).
Modeling study
In order to explore scenarios and hypotheses beyond the constraints of our experiment, we applied an individual-based population model of our predator-prey-toxicant system. Submodels were developed, parameterized and tested independently of the current study28,33,41,57. For modeling details see Appendix S2 in Supporting Information.
We ran the model for two different scenarios, (I) in accordance with the settings of our laboratory experiment as described above, applying nominal chemical concentrations and (II) in larger environments that were able to carry more than a single backswimmer under control conditions in order to examine the effect of predator size, predation intensity and the presence of kairomones on Daphnia population dynamics. For the latter scenario, simulation settings differed from the experiments as follows. We used 150 l environments and supplied Daphnia populations with food at a rate of 25 mgC d−1. Notonectids were placed into the systems on simulation day 40, and 2-day nonylphenol pulse exposures were initiated on day 60. For simulations we used the predator profiles of the 1st and 5th instar backswimmers and assumed size to be constant. We calculated the ratio of small daphnids (neonates + juveniles) and adults as a measure for population size-structure on simulation day 63 and the extinction probability, i.e. the fraction of populations that went extinct in 100 Monte-Carlo simulations, on simulation day 70.
Author Contributions
A.G. designed and carried out the experiment, performed modeling work and analyzed data, A.Z. analyzed chemical concentrations, T.G.P. designed the experiment and performed modeling work, A.G. and V.G. wrote the first draft of the manuscript and all authors contributed substantially to revisions.
Supplementary Material
Supplementary information
Acknowledgments
We thank Stefan Rhiem for laboratory assistance and Andrew Brown for proof reading. Kristian Syberg provided helpful comments on the draft manuscript. AG has been financially supported by the European Union under the 7th Framework Programme (project acronym CREAM, contract number PITN-GA-2009-238148).
References
- Kundi M., Hardell L., Sage C. & Sobel E. Electromagnetic Fields and the Precautionary Principle. Environ. Health. Perspect. 117, A484–A485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R. Silent spring. (Houghton Mifflin, Boston, Massachusetts, 1962). [Google Scholar]
- Hastings A. Transient dynamics and persistence of ecological systems. Ecol. Lett. 4, 215–220 (2001). [Google Scholar]
- Carpenter S. R., Walker B., Anderies J. M. & Abel N. From metaphor to measurement: resilience of what to what? Ecosystems 4, 765–781 (2001). [Google Scholar]
- Simpson C. & Kiessling W. The role of extinction in large-scale diversity-stability relationships. Proc. R. Soc. B 277, 1451–1456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D. Mobile phone-induced honeybee worker piping. Apidologie 42, 270–279 (2011). [Google Scholar]
- Whitehorn P. R., O'Connor S., Wackers F. L. & Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012). [DOI] [PubMed] [Google Scholar]
- Liess M. Population response to toxicants is altered by intraspecific interaction. Environ. Toxicol. Chem. 21, 138–142 (2002). [PubMed] [Google Scholar]
- Coors A. & De Meester L. Synergistic, antagonistic and additive effects of multiple stressors: predation threat, parasitism and pesticide exposure in Daphnia magna. J. Appl. Ecol. 45, 1820–1828 (2008). [Google Scholar]
- Marcogliese D. J. et al. Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat. Toxicol. 91, 126–134 (2009). [DOI] [PubMed] [Google Scholar]
- Hanazato T. & Dodson S. I. Complex effects of a kairomone of Chaoborus and an insecticide on Daphnia pulex. J. Plankton. Res. 14, 1743–1755 (1992). [Google Scholar]
- Hanazato T., Fueki K. & Yoshimoto M. Fish-induced life-history shifts in the cladocerans Daphnia and Simocephalus, are they positive or negative responses? J. Plankton. Res. 23, 945–951 (2001). [Google Scholar]
- Schulz R. & Dabrowski J. M. Combined effects of predatory fish and sublethal pesticide contamination on the behavior and mortality of mayfly nymphs. Environ. Toxicol. Chem. 20, 2537–2543 (2001). [DOI] [PubMed] [Google Scholar]
- Beketov M. A. & Liess M. The influence of predation on the chronic response of Artemia sp. populations to a toxicant. J. Appl. Ecol. 43, 1069–1074 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. D. & Banken J. A. Importance of population structure at the time of toxicant exposure. Ecotoxicol. Environ. Safety. 42, 282–287 (1999). [DOI] [PubMed] [Google Scholar]
- Stark J. D. & Banks J. E., Acheamponga S. Estimating susceptibility of biological control agents to pesticides: influence of life history strategies and population structure. Biological Control 29, 392–398 (2004). [Google Scholar]
- Dodson S. I. & Havel J. E. Indirect prey effects - some morphological and life-history responses of Daphnia pulex exposed to Notonecta undulata. Limnol. Oceanogr. 33, 1274–1285 (1988). [Google Scholar]
- Coors A., Hammers-Wirtz M. & Ratte H. T. Adaptation to environmental stress in Daphnia magna simultaneously exposed to a xenobiotic. Chemosphere 56, 395–404 (2004). [DOI] [PubMed] [Google Scholar]
- Black A. R. Predator-induced phenotypic plasticity in Daphnia pulex - life-history and morphological responses to Notonecta and Chaoborus. Limnol. Oceanogr. 38, 986–996 (1993). [Google Scholar]
- Milbrink G. & Bengtsson J. The impact of size-selective predation on competition between two Daphnia species: A Laboratory Study. J. Anim. Ecol. 60, 1009–1028 (1991). [Google Scholar]
- Liess M. & Foit K. Intraspecific competition delays recovery of population structure. Aquat. Toxicol. 97, 15–22 (2010). [DOI] [PubMed] [Google Scholar]
- Brooks J. L. & Dodson S. I. Predation, body size and composition of plankton. Science 150, 28–35 (1965). [DOI] [PubMed] [Google Scholar]
- Campbell C. E. Prey selectivities of three spine sticklebacks (Gasterosteus aculeatus) and phantom midge larvae (Chaoborus spp) in Newfoundland lakes. Freshwater Biol. 25, 155–167 (1991). [Google Scholar]
- Newman R. M. & Waters T. F. Size-selective predation on Gammarus pseudolimnaeus by trout and sculpins. Ecology 65, 1535–1545 (1984). [Google Scholar]
- Hansson L. A. et al. Consequences of fish predation, migration, and juvenile ontogeny on zooplankton spring dynamics. Limnol. Oceanogr. 52, 696–706 (2007). [Google Scholar]
- Reynolds J. G. & Geddes M. C. Functional response analysis of size-selective predation by the notonectid predator Anisops deanei (Brooks) on Daphnia thomsoni (Sars). Aust. J. Marine Freshwater Res. 35, 725–733 (1984). [Google Scholar]
- Dieguez M. C. & Gilbert J. J. Predation by Buenoa macrotibialis (Insecta, Hemiptera) on zooplankton: effect of light on selection and consumption of prey. J. Plankton. Res. 25, 759–769 (2003). [Google Scholar]
- Gergs A. & Ratte H. T. Predicting functional response and size selectivity of juvenile Notonecta maculata foraging on Daphnia magna. Ecol. Model. 220, 3331–3341 (2009). [Google Scholar]
- Yen J. Effects of prey concentration, prey size, predator life stage, predator starvation, and season on predation rates of the carnivorous copepod Euchaeta elongata. Mar. Biol. 75, 69–77 (1983). [Google Scholar]
- Paradis A. R., Pepin M. & Pepin P. Disentangling the effects of size-dependent encounter and susceptibility to predation with an individual-based model for fish larvae. Can. J. Fish. Aquat. Sci. 56, 1562–1575 (1999). [Google Scholar]
- Scharf F. S., Juanes F. & Rountree R. A. Predator size-prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 208, 229–248 (2000). [Google Scholar]
- Scott M. A. & Murdoch W. W. Selective predation by the backswimmer. Notonecta. Limnol. Oceanogr. 28, 352–366 (1983). [Google Scholar]
- Gergs A., Hoeltzenbein N. I. & Ratte H. T. Diurnal and nocturnal functional response of juvenile Notonecta maculata considered as a consequence of shifting predation behaviour. Behav. Process. 85, 151–156 (2010). [DOI] [PubMed] [Google Scholar]
- Giller P. S. The natural diet of the Notonectidae - field trials using electrophoresis. Ecol. Entomol. 11, 163–172 (1986). [Google Scholar]
- Murdoch W. W. & Scott M. A. Stability and extinction of laboratory populations of zooplankton preyed on by the backswimmer Notonecta. Ecology 65, 1231–1248 (1984). [Google Scholar]
- McArdle B. H. & Lawton J. H. Effects of prey-size and predator-instar on the predation of Daphnia by Notonecta. Ecol. Entomol. 4, 267–275 (1979). [Google Scholar]
- Werner E. & Peacor S. A review of trait-mediated indirect interactions in ecological communities. Ecology 84,1083–1100 (2003). [Google Scholar]
- Creel S. & Christianson D. Relationships between direct predation and risk effects. Trends Ecol. Evol. 23, 194–201 (2008). [DOI] [PubMed] [Google Scholar]
- Cox E. J., Naylor C., Bradley M. C. & Calow P. Effect of differing maternal ration on adult fecundity and offspring size in laboratory cultures of Daphnia magna Straus for ecotoxicological testing. Aquat. Toxicol. 24, 63–74 (1992). [Google Scholar]
- Glazier D. S. Effects of food, genotype, and maternal size and age on offspring investment in Daphnia magna. Ecology 73, 910–926 (1992). [Google Scholar]
- Preuss T. G., Hammers-Wirtz M., Hommen U., Rubach M. N. & Ratte H. T. Development and validation of an individual based Daphnia magna population model: The influence of crowding on population dynamics. Ecol. Model. 220, 310–329 (2009). [Google Scholar]
- Burns C. W. Effects of crowding and different food levels on growth and reproductive investment of Daphnia. Oecol. 101, 234–244 (1995). [DOI] [PubMed] [Google Scholar]
- Preuss T. G. Ökotoxikologische Charakterisierung von Nonylphenol Isomeren. (Shaker, Aachen, Germany, 2007). [Google Scholar]
- Agatz A., Hammers-Wirtz M., Gabsi F., Ratte H. T., Brown C. D. & Preuss T. G. Promoting effects on reproduction increase population vulnerability of Daphnia magna. Environ. Toxicol. Chem. 31, 1604–10 (2012). [DOI] [PubMed] [Google Scholar]
- Takahashi H. & Hanazato T. Synergistic effects of food shortage and an insecticide on a Daphnia population: rapid decline of food density at the peak of population density reduces tolerance to the chemical and induces a large population crash. Limnology 8, 45–51 (2007). [Google Scholar]
- McCauley E. & Murdoch W. W. Cyclic and stable populations: plankton as paradigm. Am. Nat. 129, 97–121(1987). [Google Scholar]
- Greco W. R., Bravo G., Parsons J. C. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47, 331–85 (1995). [PubMed] [Google Scholar]
- Scheffer M., Carpenter S. R. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends. Ecol. Evol. 18, 648–656 (2003). [Google Scholar]
- Caswell H. Matrix Population Podels: Construction, Analysis and Interpretation. (Sinauer Associates, Sunderland, Massachusetts, 2001). [Google Scholar]
- Grimm V. & Railsback S. F. Individual-based Modelling and Ecology. (Princeton University Press, Princeton, New Jersey, 2005). [Google Scholar]
- Forbes V. E. & Calow P. Population growth rate as a basis for ecological risk assessment of toxic chemicals. Phil. Trans. R. Soc. 357, 1299–1306 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. C. M., Arts G. H. P., Maltby L. & Van den Brink P. J. Aquatic risks of pesticides, ecological protection goals, and common aims in European Union legislation. Integr. Environ. Assess. Manage. 2, 20–46 (2006). [Google Scholar]
- Stark J. D. & Banks J. E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 48, 505–519 (2003). [DOI] [PubMed] [Google Scholar]
- Siehoff S., Hammers-Wirtz M., Strauss T. & Ratte H. T. Periphyton as alternative food source for the filter-feeding cladoceran Daphnia magna. Freshwater Biol. 54, 15–23 (2009). [Google Scholar]
- Elendt B. P. & Bias W. R. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing - effects of the optimization of culture conditions on life-history parameters of Daphnia magna. Water Research 24, 1157–1167 (1990). [Google Scholar]
- Russ A. S., Vinken R., Schuphan I. & Schmidt B. Synthesis of branched paranonylphenol isomers: Occurrence and quantification in two commercial mixtures. Chemosphere 60, 1624–1635 (2005). [DOI] [PubMed] [Google Scholar]
- Gergs A. Modelling Foraging Behaviour in the Insect Predator Notonecta maculata using the Individuals Approach. PhD Thesis (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information