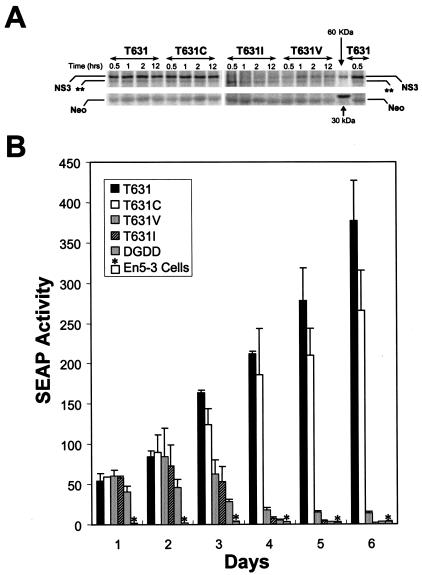
FIG. 6.
The T631C mutation degrades the replication capacity of a subgenomic replicon derived from the genotype 1b HCV-N virus that induces the expression of SEAP proportional to the intracellular abundance of replicon RNA. (A) In vitro translation reactions were programmed with replicon RNAs encoding the wild-type NS3-4A junction (NTat-T631) or cysteine, valine, or isoleucine replacements of the P1 threonine normally present at residue 631 and incubated for 0.5, 1, 2, or 12 h, followed by separation of the products by SDS-PAGE and autoradiographic visualization of the mature NS3 product of the NS3-4A cleavage. (Top) Translated mature NS3 protein. The band marked ** corresponds in apparent molecular mass to the predicted NS5B product. (Bottom) Neomycin phosphotransferase product (Neo) expressed from the upstream cistron of the replicon RNAs, which served as a translation control in these experiments. The positions of 30- and 60-kDa markers are shown. The T631 and T631C products underwent efficient processing at the NS3-4A junction, while the T631V and T631I products did not. (B) Transient-transfection assays demonstrate that the NTat-T631 replicon bearing the wild-type NS3-4A junction has greater replication capacity than NTat-T631C and much greater replication capacity than NTat-T641V and -T631I (whose replication capacity is indistinguishable from that of replication-defective NS5B deletion mutant ΔGDD). En5-3 cells were transfected with equal amounts of the indicated replicon RNAs by electroporation and then cultured in the absence of G418 selection for 6 days. Supernatant culture media were sampled at 24-h intervals and assayed for SEAP activity as a measure of the intracellular replicon RNA abundance (see Materials and Methods). Results shown represent the mean SEAP activities present in supernatant fluids at the times indicated. Error bars represent the ranges of values in replicate transfection experiments using RNA transcripts produced in separate reactions. T631, NTat-T631. *, SEAP expressed by nontransfected En5-3 cells.