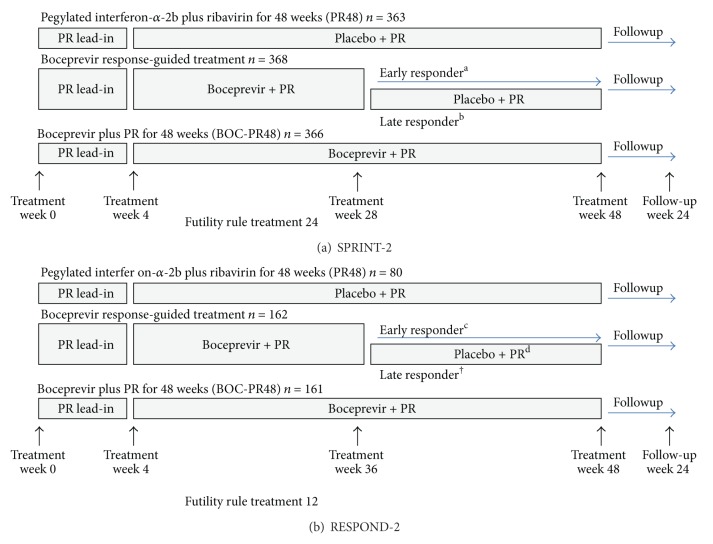
Figure 2.
Phase III trials of boceprevir in patients with hepatitis C genotype-1 infection. (a) SPRINT-2 trial in previously untreated patients. (b) RESPOND-2 trial for previously treated patients; patients were partial responders and relapsers and null-responders. PR: pegylated interferon-α-2b 1.5 μg/kg per week plus weight-based ribavirin 600–1400 mg per day. BOC: boceprevir 800 mg every 8 h. aHepatitis C RNA treatment weeks 8–24 undetectable. bHepatitis C RNA treatment week 8 detectable, treatment week 24 undetectable. cHepatitis C RNA treatment weeks 8–12 undetectable. dHepatitis C RNA treatment week 8 detectable, treatment week 12 undetectable. Excerpted from Pearlman [11].