Figure 5.
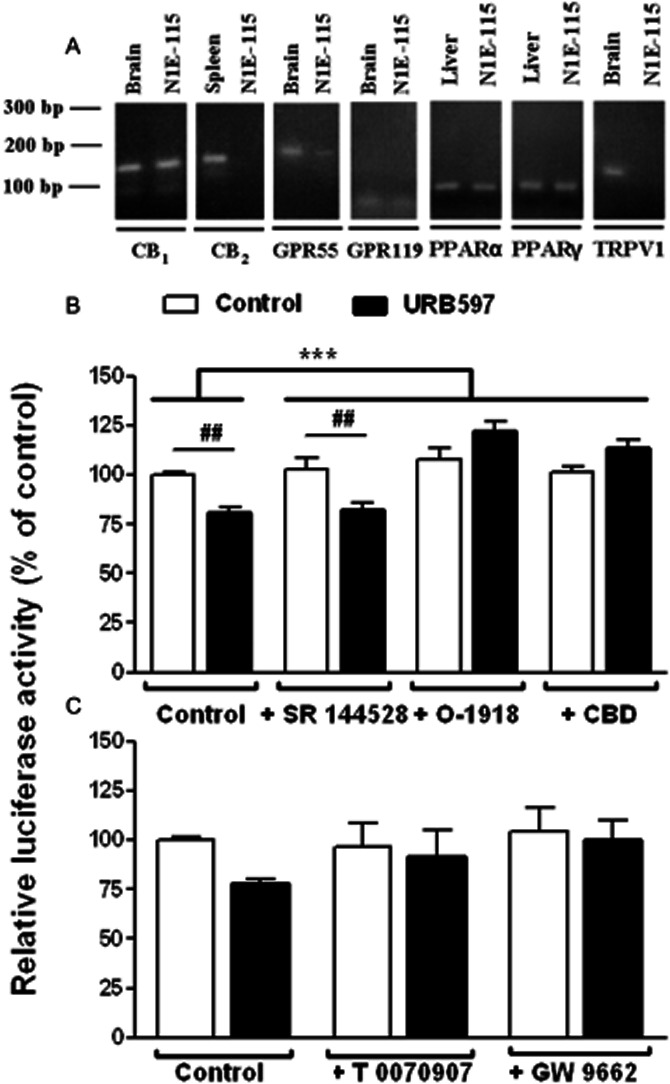
Involvement of non-CB1/non-CB2 cannabinoid receptors and PPARs in the URB597-mediated regulation of TH promoter activity. (A) RT-PCR was performed on N1E115 cell mRNA extracts with specific primers targeting the different endocannabinoid molecular targets. RT-PCR yielded the predicted amplification products for CB1, GPR55 and GPR119 receptors as well as for PPARα and PPARγ. No bands were detected for CB2 cannabinoid receptor and TRPV1 despite a correct amplification in spleen and brain tissues respectively. Negative controls were performed without any DNA template (not shown). The influence of SR 144528 at 1 μM (B), O-1918 at 30 μM (B), CBD at 10 μM (B) or PPAR antagonists (C) on luciferase activity was examined using transfected N1E115 cells carrying pTH250-Luc. Cells were concomitantly treated with the indicated antagonists and 0.1 μM URB597. The tested PPAR antagonists were T 0070907 (PPARγ antagonist, 10 μM) or GW 9662 (PPARγ/PPARα antagonist, 20 μM). Results are given as the percentages of relative luciferase activity (firefly luciferase relative to Renilla luciferase) relative to control values. Data are means with SEM values of at least three experiments performed in triplicate. Two-way ANOVA indicated a general effect of some of the antagonists (***P < 0.0001, f = 18.76, residual d.f. = 18) with a positive interaction (***P < 0.0001, f = 12.13) for (B). ##P < 0.01, as indicated for treated cells and determined by Bonferroni post-test. In (C), Bartlett's statistic revealed that variances significantly differ between these groups. Despite a strong trend (P = 0.062 for a general effect of URB597 in two-way ANOVA), statistical significance was not reached using the non-parametric Kruskal–Wallis test effect.
