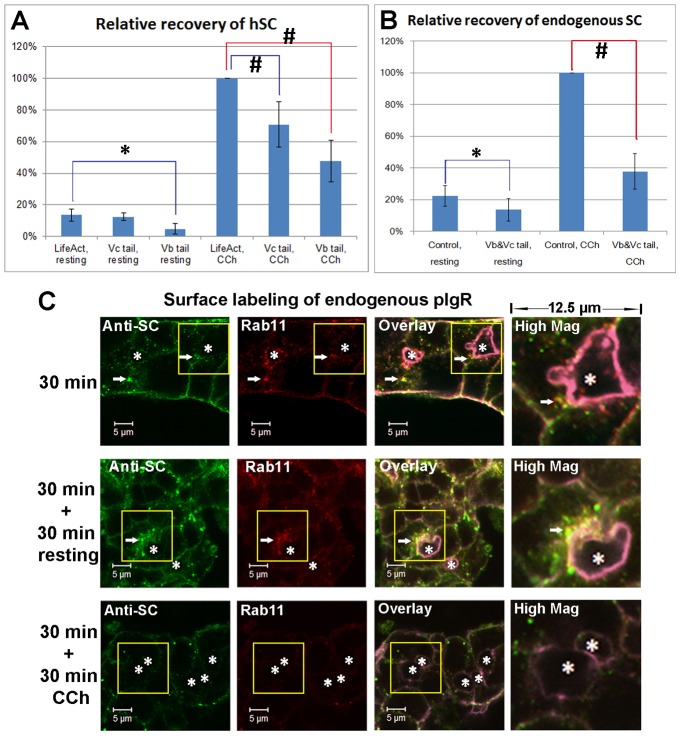
Fig. 7.
CCh accelerates the SC release on the apical membrane of LGAC acini. (A) Quantification of hSC released into culture supernatant by LGACs co-transduced with Ad hpIgR-EGFP, Adeno-X Tet-On® and rAVCMVLifeAct–TagRFP, Ad EGFP-myosin Vc tail or Ad mCherry-myosin Vb tail were cultured overnight with doxycycline. LifeAct-TagRFP is a control for EGFP myosin-Vc tail or mCherry-myosin-Vb tail transduction. (B) Quantification of endogenous rabbit SC released into culture supernatant. LGACs were co-transduced with rAVCMVLifeAct–TagRFP and Ad syncollin-GFP (control combination), or with Ad EGFP-myosin Vc tail and Ad mCherry-myosin Vb tail (experimental treatment combination). For both A and B, the net release of hSC or endogenous rabbit SC into the culture supernatant during a 30-minute treatment was quantified by western blotting, as described in Materials and Methods. n = 5; #P<0.05, one-sample Wilcoxon signed rank test; *P<0.05, Student's t-test. (C) Non-transduced LGACs were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C for 30 minutes. Cells were then treated with or without 100 µM CCh for an additional 30 minutes. Cells were fixed at the time points indicated, permeabilized, and labeled with primary mouse anti-Rab11 antibody, secondary Alexa Fluor®-488-conjugated donkey anti-sheep and Alexa Fluor®-568 goat anti-mouse antibodies, and Alexa Fluor®-647-conjugated phalloidin. Actin labeled with Alexa Fluor®-647-conjugated phalloidin is displayed in purple in overlay and high magnification images. White arrows, colocalization; *, lumena. Scale bars: 5 µm.