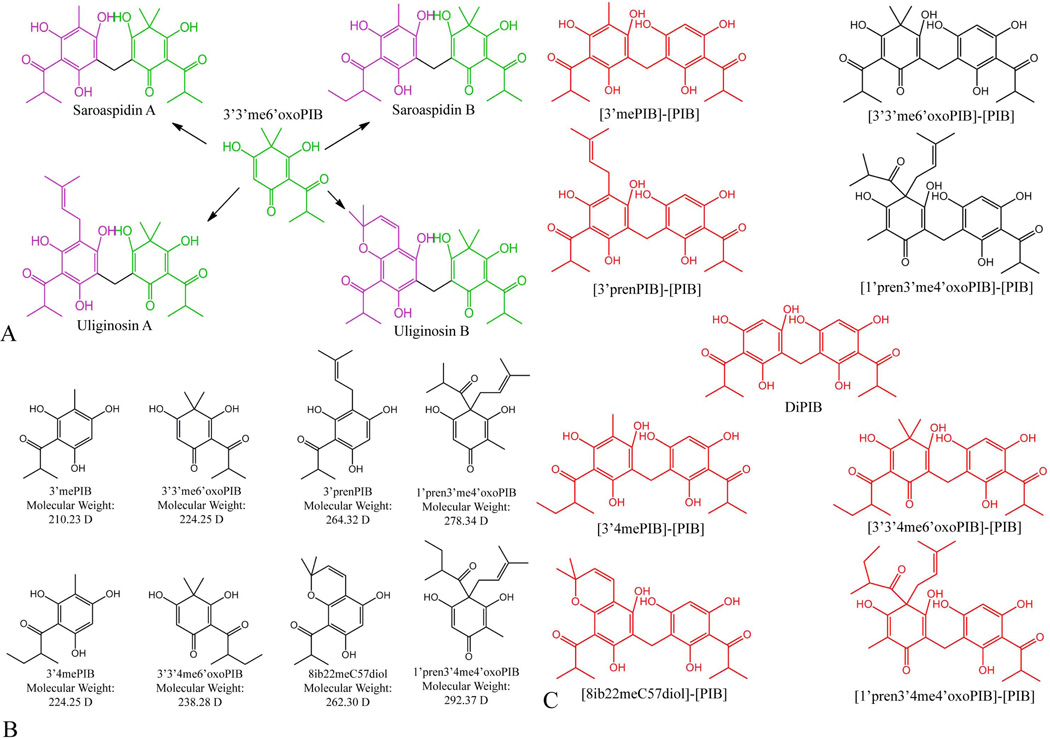
Figure 2.
Diacylphloroglucinols in Hypericum gentianoides. A) Each diacylphloroglucinol identified in H. gentianoides (see Table 1) is composed of one phloroglucinol (purple) and one filicinic acid (green); many of these diacylphloroglucinols share a common phloroglucinol or filicinic acid. For example, Saroaspidin A, Saroaspidin B, Uliginosin A, and Uliginosin B, all share the filicinic acid 3’,3’-dimethyl-6’-oxo-phlorisobutyrophenone (3’3’me6’oxoPIB). B) Four PIB derivatives from H. gentianoides identified by Q-TOF. Methylation and/or prenylation of PIB would give rise to each of these compounds. Additional methylation and/or cyclization of the resultant compounds would give rise to four more monomers, creating a pool of eight monomers sufficient to explain the synthesis of all nine highly accumulated acylphloroglucinols, without any post-dimerization modifications. C) Nine diacylphloroglucinols would be possible if diacylphloroglucinol biosynthesis occurred via a “diacylphloroglucinol decoration” route. In black are compounds that were identified through our Q-TOF analyses, in red are compounds that were not detected in our Q-TOF analysis of H. gentianoides extracts. The fully undecorated dimer (diPIB), and six of the eight partially decorated dimers that would be required if synthesis followed the “diacylphloroglucinol decoration” route were not detected. We assume the two partially decorated dimers present in H. gentianoides are 1) degradation products of diacylphloroglucinol end products or 2) products resulting from dimerization of a decorated monomer to a PIB molecule.