Abstract
Background:
Breast cancer remains a substantial health concern in Iran due to delay and late stage at diagnosis and treatment. Despite the potential benefits of mammography screening for early detection of breast cancer, the performance of this screening among Iranian women is low. For planning appropriate intervention, this study was carried out to identify mammography rates and explore determinants of mammography screening behavior in females of Isfahan, Iran.
Materials and Methods:
In this population-based study, 384 women of 40 years and older were interviewed by telephone. The Farsi version of Champion's Health Belief Model scale (CHBMS) was used to examine factors associated with mammography screening. The obtained data were analyzed by SPSS (version 16.0) using statistical Chi-square, Fisher Exact test, t-test and multiple logistic regression model to identify the importance rate of socio-demographic and Health Belief Model (HBM) variables to predict mammography screening behavior. In all of tests, the level of significant was considered a = 0.05.
Results:
Mean age ± SD of women was 52.24 ± 8.2 years. Of the 384 participants, 44.3% reported at least one mammogram in their lifetime. Logistic regression analysis indicated that women were more likely to have mammography if they heard/read about breast cancer (OR = 4.17, 95% CI 2.09, 8.34), menopause in lower age (OR = 0.2, 95% CI 0.87, 0.99) and history of breast problem (OR = 0.9, 95% CI 0.12, 0.32). Also, women who perceived more benefits of mammography (OR = 1.84, 95% CI 1.63, 2.09), fewer barriers of mammography (OR = 0.91, 95% CI 0.86, 0.96) and had more motivation for health (OR = 0.94, 95% CI 0.89, 1) were more likely to have mammography.
Conclusion:
The findings indicated that the rate of mammography screening among women in Isfahan province is low and highlights the need for developing a comprehensive national breast cancer control program, which should be considered as the first priority for healthcare providers. Also, identification of these factors can help to design an appropriate educational intervention that focuses on benefits of mammography screening, decreasing changeable barriers, improving access to mammography, increasing health motivation, promoting perceived self-efficacy and mammography adherence.
Keywords: Breast cancer, health belief model, mammography screening, women
INTRODUCTION
In recent years, breast cancer remains a substantial health concern because it is the most frequently diagnosed type of cancer among women throughout the world.[1,2] International statistics reveals incremental incidence of breast cancer and it is growing more quickly in low incidence rate communities.[3,4] The American Cancer Society estimated that 226,870 new cases of invasive breast cancer and 39,920 deaths (39,510 women and 410 men) occurred in the United States in 2012.[5]
In Asian countries, especially, Islamic Republic of Iran, this increase in incidence rate has provoked breast cancer as one of the most common cancers among Iranian women.[6] According to the literature, there are few reports about breast cancer in Iran. The National Cancer Registry reports from 2003 to 2006 showed that the numbers and rates of breast cancer among women are rising sharply, as new cases of breast cancer and Age-Standardized Rate (ASR) increased nearly two-fold from 2003 (3,250 cases, 12.40 per 100,000) to 2006 (6,456 cases, 25.06 per 100,000).[7] The latest formal report from the Cancer Institute of Iran showed that breast cancer constitutes 25% of all cancers in Iranian females and the most in age group of 35–44 years.[8] On the whole, breast cancer encompasses 41.24% and 93.25% of women cancers in our country and Isfahan, respectively.[9] Results of an epidemiological review on breast cancer in Iran demonstrated that 82% of women are diagnosed with advanced stage breast cancer (18% Stage II and 77% Stage III).[6]
Diagnosis of breast cancer in the late stage and delay in prompt treatment of this disease is related to lower survival rate.[10,11] If breast cancer were detected at an early stage, survival rate could be improved to as high as 95%.[6] Several surveys have indicated decrease in breast cancer-related mortality among women since 1990, the result of both early detection and improvement in treatment.[5,12,13] Although breast cancer incidence rate in Iran is still low in comparison with developed countries (25/100,000 in Iran compared with 140.8/100,000 in U.S.), Iranian females have a higher risk of breast cancer death in contrast with these countries.[7] Mousavi et al. study showed that over 36% tumors occur in women who are under 40 years old.[14] In reality, one of every four Iranian female receives their diagnosis when the disease has already spread.[10]
As a result of having the opportunity to be diagnosed in preclinical stage, reducing breast cancer morbidity and mortality depends on secondary prevention.[13,15] The object of breast cancer screening is to combine early diagnosis with effective treatment to decrease in both morbidity and mortality rate.[16] Mammography, clinical breast examination (CBE), and breast self examination (BSE) are generally used as secondary preventive methods.[1,17]
Ministry of Health and Medical Education in Iran recommends the following schedule for breast cancer screening: (1) Mammography: women 40 years and older should have a mammogram every year, (2) CBE: women between 20 and 40 years should have a CBE every 3 years and after the age 40 years a yearly CBE by a health professional, (3) BSE: women 20 years of age and older should perform a BSE every month.[18] Also, The American Cancer Society, the American Medical Association (AMA) and the American College of Obstetricians and Gynecologist (ACOG) recommend that women aged 40 and older should have screening mammography annually and going on it as long as they are in good health.[5,13,19]
At present, population-based breast cancer screening techniques, especially mammography, are presented in the majority of western countries. This program is present only in two countries of Asia: Japan from 1987 and Singapore from 2003.[20] The sensitivity and specificity of mammography screening is estimated to be 39% to 89% and 94% to 97% respectively.[21] Population-based screening program in U.S. in 463,372 mammography screenings showed a general sensitivity of 75% and specificity of 92.3%.[22]
Despite the potential benefits of mammography screening for breast cancer in women aged 40 years and older, the finding of studies (few studies) revealed that the performance of breast cancer screening among Iranian women is low (1.3% to 30.5%).[8,23,24,25,26,27] Therefore, maximizing participation of women in our community in mammography screening must be considered a fundamental priority. Previous studies have shown that mammography screening is associated with perceptions of risk, benefits, and barriers by using a reasoning process that encompasses personal and social effects and attitudes.[19,28]
Many mammography studies applied Health Belief Model (HBM) to distinguish factors related to mammography.[26,28,29,30,31] The most important barriers reported pain of mammography, embarrassment, not having a doctor recommending it, and barriers related to the health care system.[29,32,33,34] Effectiveness factors in having a mammography screening among Iranian women are unknown. Therefore, in this study, the HBM has been applied as the theoretical framework to determine variables related to mammography screening performance.
The HBM is by far the most commonly used theory in health education and health promotion.[35,36] This model is a psychological model, which originally advanced in the 1950s as a way to explain why medical screening programs offered by U.S Public Health Services, especially tuberculosis, were not very successful.[35,36] The basic concept of HBM is that health behavior is determined by personal beliefs or perceptions about a disease and the strategies available to reduce its action.[35,36] The HBM has been extensively used to investigate beliefs linked to breast cancer screening behaviors such as mammography.[19,37,38,39]
The HBM comprises 6 concepts: (1) Perceived susceptibility (women believe that they have the chances of getting breast cancer), (2) perceived severity (belief that breast cancer is a serious and fatal disease), (3) Perceived benefits (perceive more benefits from mammography screening), (4) Perceived barriers (perceive barriers to having a mammography), (5) Health motivation (women's motivation related to performing the health behaviors) and (6) Self-efficacy: (level of women's confidence about successfully having a mammography).[37,40,41,42] Many researchers have introduced the predictive power of the HBM in breast cancer screening behavior, particularly mammography.[34,43,44]
In our review of the scientific literature, few studies have examined factors related to mammography screening behavior in Iran, and these studies are targeted towards special groups of women.[26,32,45] Also, no research has investigated these variables in Isfahan women. Given the high rate of breast cancer in Isfahan,[9] determining these factors can provide useful framework for designing appropriate interventions to promote mammography screening in Isfahanian women. Therefore, the purpose of this study was to identify mammography rates and explore factors associated with mammography screening behavior by employing components from HBM among females of Isfahan, Iran.
MATERIALS AND METHODS
Study design and sample
This study was a population-based survey in which the factors related to mammography screening behavior on the basis of HBM were investigated. The sample population of this research was women aged 40 years and older in Isfahan, a city located in the central region of Iran. Inclusion criteria for participation in this study included being a Isfahanian women aged 40 years and older, no personal history of breast cancer, willingness to participant in the survey, and being able to speak. Therefore, we excluded females with a history of breast cancer and women <40 years old.
Telephone interviews were carried out with 348 women >40 years of age randomly selected from the communication center of Isfahan, Iran. At first, 2500 immobile telephone numbers were acquired from Isfahan communication center, which were randomly chosen from all of telephone centers of Isfahan city. Telephone interviews were accomplished over a 14-week period from March 2011 through June 2011. One female PhD student of health education conducted all telephone interviews. At first, the interviewer briefly explained the purpose of the survey and how their response may help future planning for women's health. Also, confidence was given to each person including its anonymous and voluntary nature. Then, data was collected from those who verbally consented to participate. Each telephone interview lasted about 20–45 minutes. From a total of 2500 telephone calls, there was no response from 985 (39.4%) telephone calls, 82 (3.28%) of the telephone numbers were blocked, 196 (7.8%) were not women, 648 (25.92%) women were in the unsuitable age group (<40 years old), and 589 (23.56%) contained a women in the appropriate age group (>40 years old) which 205 women were not eligible to be interviewed in the study due to unwillingness to participate or having personal history of breast cancer [Figure 1]. For each telephone number for which there was no response or where the women were absent, telephone dialing was repeated a total of three times at different times of the day with at least one time being the weekend. As a result, 348 women were interviewed by telephone. The Ethical Committee of Isfahan Medical University approved the study.
Figure 1.

Flow chart of study participants
Instruments
Data collection instruments include four sections:
Socio-demographic questions, knowledge about breast cancer and mammography, screening behavior of mammography, and the HBM scale.
Information such as age, marriage age, age of first birth, level of education, current marital status, number of children, breastfeeding duration, menopausal status, health insurance coverage, monthly household income, having first-degree relatives with history of breast cancer, and personal history of breast problems provided the socio-demographic variables.
To measure the women's level of knowledge of breast cancer, we used 11 questions in a checklist, which was developed by the researchers based on an extensive review of the published studies. These questions were about breast cancer risk factors (6 items), signs and symptoms of breast cancer (1 item), early detection of breast cancer, and mammography screening (4 items). For all of the questions, except the symptom of breast cancer, the answers were “true”, “false,” and “don't know.” For each question, true response was scored 1, false and don't know = 0. Thus, for each woman, a score between 0 and 11 was computed.
Mammography screening behavior was assessed using three questions. These questions were included: (1) “Have you ever had a mammogram (yes/no)” (2) If yes, “number of mammography,” and (3) “The time of last mammography screening.”
To assess beliefs and attitudes about breast cancer and mammography, we used the Champion Health Belief Model Scale (CHBMS). It is a commonly used scale to measure HBM components. The CHBMS was developed in 1984 and it has been revised three times.[46] The latest version of the CHBMS was adapted for Iranian use by Taymoori and Berry.[47] In this study, we used the Farsi version of the CHBMS after obtaining allowance from authors. This scale includes 61 items with eight subscales of which six subscales were used in this survey.
Perceived susceptibility:
This subscale measures a woman's perceptions about her chances of getting breast cancer by three items including: (1) “It is likely that I will get breast cancer,” (2) “My chances of getting breast cancer in the next few years are great,” and (3) “I feel I will get breast cancer sometime during my life” (Cronbach a = 0/82).
Perceived severity
Perceived severity was assessed using a seven-item scale about a female's beliefs with reference to severity of breast cancer: (1) “The thought of breast cancer scares me,” (2) “When I think about breast cancer, my heart beats faster,” (3) “I am afraid to think about breast cancer,” (4) “Problems I would experience with breast cancer would last a long time,” (5) “Breast cancer would threaten my relationship with my husband,” (6) “If someone had breast cancer, her whole life would change,” and (7) “If someone developed breast cancer, she would not live longer than 5 years” (Cronbach a = 0/84).
Health motivation
Health motivation was measured by the following seven-item scale: (1) “I want to discover health problems early,” (2) “Maintaining good health is extremely important to me,” (3) “I search for new information to improve my health,” (4) “I feel it is important to carry out activities that will improve my health,” (5) “I eat well-balanced meals,” (6) “I exercise at least 3 times a week,” and (7) “I have regular check-up even when I am not sick” (Cronbach a = 0/77).
Mammography benefits
Mammography benefits were determined using a six-item scale: (1) “When I get a recommended mammogram, I feel self-satisfied,” (2) “If I get a mammogram and nothing is found, I don't worry as much about breast cancer,” (3) “Having a mammogram will help me find breast lumps early,” (4) “Having a mammogram will decrease my chances of dying from breast cancer,” (5) “If I find a lump through a mammogram, my treatment for breast cancer may not be as bad,” and (6) “Having a mammogram is the best way for me to find a very small lump” (Cronbach a = 0/72).
Barriers of mammography
Barriers of mammography were evaluated by a 10-item scale: (1) “I am afraid to have a mammogram because I might find out something is wrong,” (2) “I am afraid to have a mammogram because I don't understand what will be done,” (3) “I don't know how to go about getting a mammogram,” (4) “Having a mammogram is too embarrassing,” (5) “Having a mammogram takes too much time,” (6) “Having a mammogram is too painful,” (7) “I cannot remember to schedule a mammogram,” (8) “I have other problems more important than getting a mammogram,” (9) “I am too old to need a routine mammogram,” and (10) “If regularly performing Breast Self Examination, don't need to have mammography.” (Cronbach a = 0/73).
Mammography self-efficacy
Mammography self-efficacy surveyed women's confidence in having mammography screening using following four-item scale: (1) “I am confident that I can have a mammogram”; (2) “I am confident that I can get a visit time from physician for mammography”; (3) “I am confident that I can regulate my time”; and (4) “I am confident that I can get a mammogram even if it would be painful” (Cronbach a = 0/84).
All items of HBM subscales have five response options ranging from strongly disagree = 1 to strongly agree = 5. Higher scores express more agreement with health beliefs except for barriers to mammography. Each subscale was calculated separately, and therefore, six different scores were obtained for each subject.
Data analyses
The obtained data were analyzed by SPSS version 16.0 (SPSS Inc, Chicago, Illinois). Descriptive analyses were used to summarize the subject's variables. Chi-square, Fisher Exact test, and t-test were used for analyses. We conducted multiple logistic regression model to identify the importance rate of socio-demographic and HBM variables to predict mammography screening behavior. In all of tests, the level of significant was considered a = 0.05.
RESULTS
Sample characteristics
The sample consisted of 384 women and their characteristics are shown in Table 1. It should be noted that a statistical formula was used to determine the sample size. The age of participants ranged from 40 to 80 years, with a mean of 52.24 years (SD = 8.21). Most participants (41.7%) were between 50 and 59 years of age. Mean of marriage age, the first pregnancy age, and menopause age was 17.16, 19.12, and 47.75 years, respectively. The majority of subjects (26.3%) had elementary graduation (grade less than 5). Most of the women (83.9%) were married, 14.1% were widowed, and 2.1% were separated or divorced. The majority of the women (78.6%) were housekeepers.
Table 1.
Sample characteristics based on telephone conversation (n = 384)
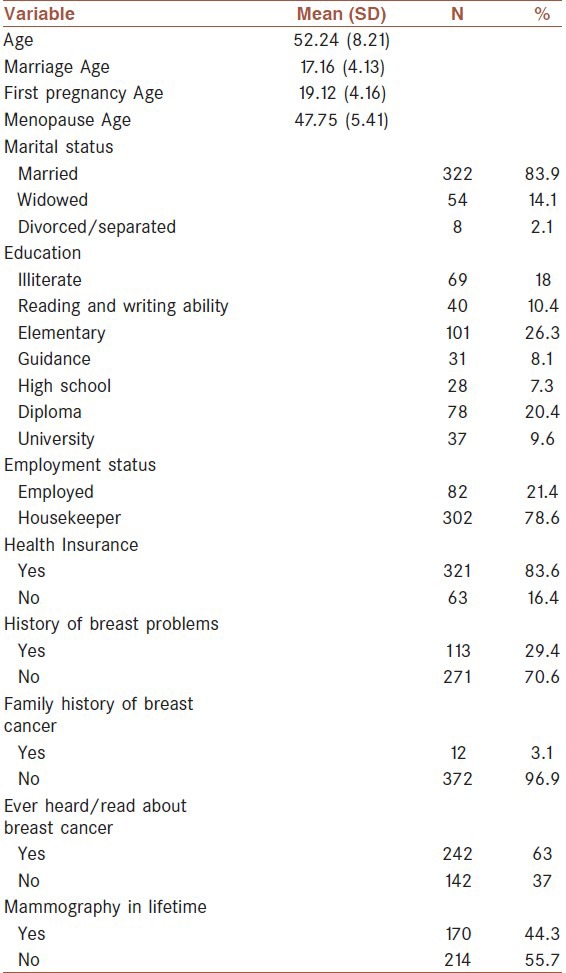
Most of the women (83.6%) had health insurance. Nearly one-third of the women (29.4%) reported history of problem or discomfort in their breast. Among these women, 45.1% expressed breast pain as the most common breast problem. Only 12 women (3.1%) had a first-degree relative who had been diagnosed with breast cancer. Forty-four percent of women reported having had at least one mammogram in their lifetime. Among these women, 14.3% stated receiving their most recent mammogram within the last 2 years, 17.4% between previous 2–5 years, and 68.2% reported longer than 5 years ago. Also, 63% of subjects stated that they heard/read information about breast cancer.
Factors related to having mammography
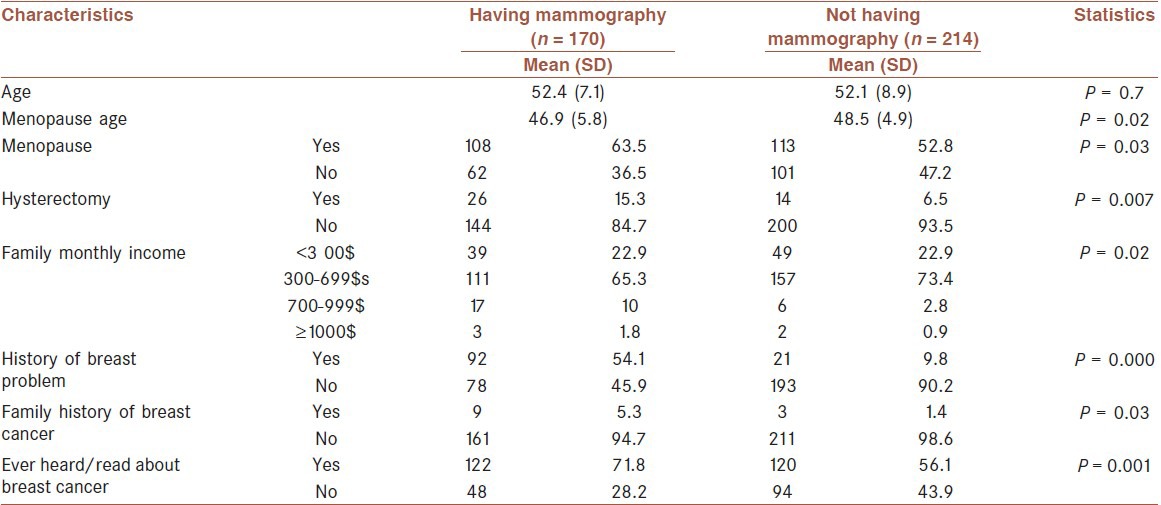
Comparison of the women's characteristics in the two groups (having mammography versus not having mammography) showed significant difference between the two groups with regard to menopause (X2 = 4.46, P = 0.03), menopause age (t = -2.24, P = 0.02), hysterectomy (X2 = 7.77, P=0.007), income level (X2 = 9.57, P = 0.02), history of breast problem (X2 = 89.5, P = 0.001), family history of breast cancer (X2 = 4.7, P = 0.03) and ever heard/read about breast cancer (X2 = 10.01, P = 0.001) [Table 2]. Age, marriage age, pregnancy age, marital status, education, employment status, and health insurance were not related variables with having mammography.
Table 2.
Bivariate predictors of having mammography (n = 384)
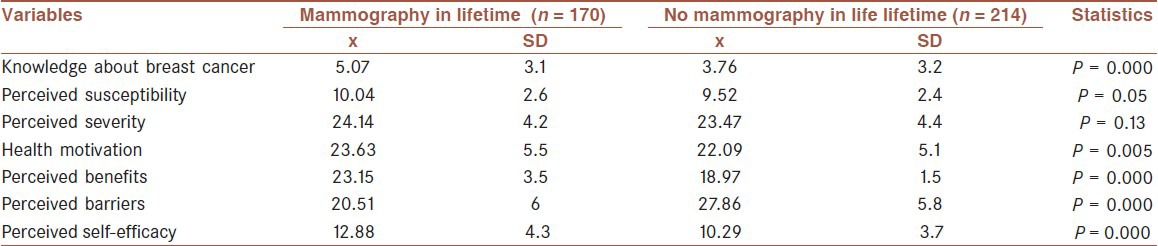
To determine the relationship between having mammography and CHBMS subscales, independent t-test was used [Table 3]. Four subscales of HBM (health motivation (t = 2.82, P = 0.005), benefits (t = 15.58, P = 0.001), barriers (t = -12.1, P = 0.001), and self-efficacy (t = 6.35,P = 0.001) for mammography screening behavior) showed significant differences between the two groups (having mammography and not having mammography). Women who previously had at least a mammogram in their lifetime had higher levels of health motivation, perceived benefits, and perceived self-efficacy to mammography screening and fewer perceived barriers to having a mammogram. No significant differences were observed between two groups with regard to perceived susceptibility (t = 1.96, P = 0.05) and perceived severity (t = 1.5, P = 0.1).
Table 3.
Mammography compliance in evaluated individuals based on knowledge and CHBMS subscale. (n = 384)
Logistic regression analysis was used to predict factors related to having mammography. The result of this analysis showed three variables with significant odds ratio [see Table 4]. Women who stated heard/read about breast cancer were over four times more likely to having mammography than those who had not (OR = 4.17, 95% CI 2.09, 8.34). Women in menopause age were near to one time more likely to have mammography than those who not having (OR = 0.2, 95% CI 0.87, 0.99) and women who reported had history of breast problem were more likely to have had mammography than those who had not (OR = 0.9, 95% CI 0.12, 0.32).
Table 4.
Logistic regression analysis of sample characteristics for having mammography
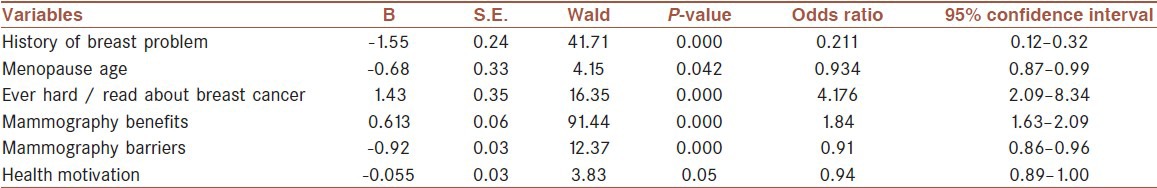
Then, the CHBMS subscales were entered into logistic regression analysis to be tested as predictor factors for having mammography. Three significant odds ratios were identified [See Table 4]. The women who perceived more benefits of mammography (OR = 1.84, 95% CI 1.63, 2.09), fewer barriers of mammography (OR = 0.91, 95% CI 0.86, 0.96), and also had more motivation for health (OR = 0.94, 95% CI 0.89, 1) were more likely to have a mammography.
DISCUSSION
Breast cancer is one of the most common cancers, which have an enormous impact on the health of Iranian women.[48] Mammography is one of the most effective methods for early detection of breast cancer. Literature published to date demonstrated that the mammography usage rate among Iranian women is lower[8,26] compared with developed countries.[33,49,50] Therefore, we need to study factors associated with mammography screening behavior among Iranian women.
In a few researches, were examined associated factors with mammography screening behavior in Iran. Also, samples of these studies were particular groups of women such as worker,[26] and women attained to hospital or health centers.[8,23,24,25,27] This study was conducted in population-based sample. The study finding indicated that 44.3% women had at least one mammogram in their lifetime. Although this rate is higher in comparison with previous studies in Iran (range 1.6–30.5%),[23,24,25,26,27] it is much lower than reported rates (ranging 15–79%) in developed countries.[28,31,33,49] However, the rate of mammography can be different in different regions. One explanation for these differences can be due to socioeconomic conditions and healthcare system. Based on WHO prediction, a breast cancer epidemic in developing countries will occur as a result of socioeconomic conditions and increase in life expectancy.[51] Despite this fact, screening rate is less than 5% in most developing countries.[26] For example, Heidari's study on women in south of Iran is surprising, considering that only 1.3% of the women had a mammography throughout their lifetime.[8]
Mousavi et al. stated that because of high prevalence of cancer risk factors, increase of numbers of aging people, and increase in life expectancy, it is expected that in future the cancer cases will rise rapidly in Iran.[52] Unfortunately, few national programs according WHO recommendation and guidelines for cancer screening are active in Iran.[53] Annual mammography has recently been recommended for women 40 years and older by Iran Ministry of Health, but any nationwide population-based breast cancer screening programs were not accomplished in Iran. Several randomized trials and population-based programs have displayed that mammography screening increases successful treatment and decreases mortality risk among women.[15,21,54,55] Most of the studies agreed that early detection is the only prevention method for breast cancer. Consequently, it is necessary to inform women regarding importance of annual mammography in decreasing mortality of breast cancer in women and how to obtain these services.
The finding of bivariate analysis for examining variables associated with having mammography revealed significant relationship with menopause age, menopause, hysterectomy, income level, history of breast problems, family history of breast cancer, and ever heard/read information about breast cancer. Excluding (Except) variables of menopause age, menopause, and hysterectomy, other mentioned findings are in accordance with results of previous studies.[34,56,57] These findings showed that women who had become menopausal in lower age or had history of hysterectomy more likely used mammogram in their lifetime, perhaps because these women more referred to physician for gynecology examination and were recommended to have mammography. Previous studies stated that recommendation by healthcare provider such as physician's recommendation and having a gynecologist as a regular physician are associated with higher mammography rate.[28,33,58,59] Currently, there isn't a comprehensive national and free program for breast cancer screening in our country, and the women require a physician's prescription for getting mammography and must pay cost of mammography themselves.
Also, this study indicated that the women with low income level were less likely than others to have mammography screening. Thus, providing low-cost or free access to mammography in women 40 years and older, especially women with low socioeconomic conditions and the women who don't have insurance, is very essential. Kerlikwoske et al. stated that providing free facilities in different parts of the community increases mammography screening in women.[60]
Contrary to other studies, our study did not demonstrate any association between age,[26] pregnancy,[30] education,[28,30,33,56] marital status,[33] health insurance,[28,33] and mammography use.
Like other studies, our study found that having a history of breast problems and having a family history of breast cancer were positively correlated to mammography use. Having history of breast cancer in first-degree relatives affects women's perception of breast cancer risk. Results of present study demonstrated that an important factor for having mammography was knowledge about breast cancer and mammography screening. The women who had ever heard/read about breast cancer were more likely to have a mammogram. This finding is consistent with previous studies that suggest knowledge about breast cancer and mammography is facilitators’ factors of mammography utilize.[28,30,61,62] Montazeri et al. suggested that “lack of knowledge about breast cancer is an important factor in Iran and there is a need for public educational program especially for less educated women.”[10]
Comparison of women's knowledge and practice in this study indicated that there is a gap between knowledge and having mammography. Although 63% of women stated that ever heard/read about breast cancer, only 44.3% of them had at least a mammogram in lifetime. It should be mentioned that although knowledge is essential for having mammography, it is not adequate. Previous studies indicated similar results.[26,63]
On the basis of the HBM, subscales of perceived susceptibility, severity, benefits, self-efficacy, and health motivation are positively related to health behavior such as mammography screening behavior and perceived barriers have negative relationship.[64] The results of this study suggest that the women aged 40 and older who perceive more benefits, more self-efficacy, and fewer barriers to mammography screening, and also women who have more motivation for health, more likely use mammography screening. It is similar to the finding of other researches that have found a positive association with perceived benefits,[26,28,34] perceived self-efficacy,[28,34,65,66] and health motivation[19] and a negative association with perceived barriers[26,28,34] and having mammography.
The top three identified benefits in the current study were feeling satisfied, reducing worry, and increasing probability of detecting tumor in early stage. Also, the most important identified barriers by women who never had a mammography included don't feel any symptoms, don't need to have mammography, other problems more important than getting a mammogram, fear of mammography, and lack of knowledge about where to get a mammogram. Other barriers such as embarrassment, lack of time, pain, and cost of mammography were significant. The majority of previous studies have found these barriers to mammography use.[26,30,33,34,67]
In contrast to HBM, perceived susceptibility and perceived severity were not significantly associated with mammography use. This finding is in agreement with findings of previous studies[26,34,62] and is in contrast with some studies.[19,28,34,40,66,68] One explanation for the inconsistent finding regarding perceived susceptibility and perceived severity may be because of lack of knowledge about breast cancer. Based on HBM, people's perception of perceived threat depends on their knowledge about the disease. Furthermore, implementing education intervention for increase of women's knowledge about breast cancer can be effective in promoting their perception from risk factors, signs, and symptoms and their susceptibility to breast cancer and benefits of mammography screening.
Finally, in this study we have found six factors as predictors of mammography screening among Isfahanian women 40 years and older: menopause age, ever heard/read about breast cancer, history of breast problems, perceived benefits, perceived barriers, and health motivation. Finding of other studies showed that social support[34] and religious beliefs[26] had significant association with mammography use. These factors were not investigated in our study and it is recommended that in further researches are surveyed.
In our study and more previous studies, women showed that the likelihood of having mammography in women is more associated with socio-demographic, cognitive, and behavioral variables; whereas mammography screening behavior also depends on healthcare system or provider. Thus, it is an important issue that must be investigated in future researches.
In conclusion, our study findings indicated that the rate of mammography screening among Iranian women is low and highlights the need for developing and implementing a comprehensive national breast cancer control program, which should be considered as the first priority for healthcare providers and health policy makers. Also, the study results can inform researches regarding associated factors with having mammography screening behavior. Identification of these factors can help to designing an appropriate educational intervention that focuses on benefits of mammography screening, decreasing changeable barriers, improving access to mammography, increasing health motivation, promoting perceived self-efficacy, and adherence mammography.
Limitation
Because the current study conducted by telephone interview, some bias may have been introduced into this study by elimination women who did not have a telephone or their telephone numbers were blocked, and recall bias, especially among older women because mammography use was examined by self-report. Despite these limitations, the telephone interview is an effective method of data collection that is used in many studies.[69,70,71,72,73]
ACKNOWLEDGMENT
This article is part of a PhD dissertation (Research project Number = 389476). The authors would like to thank the Deputy for Research of for their financial support and all the women who participated in this study.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Gursoy AA, Ylmaz F, Nural N, Kahriman I, Yigitbas C, Erdol H, et al. A different approach to breast self-examination education: Daughters educating mothers creates positive results in Turkey. Cancer Nurs. 2009;32:127–34. doi: 10.1097/NCC.0b013e3181982d7b. [DOI] [PubMed] [Google Scholar]
- 2.Seedhom AE, Kamal NN. Factors affecting survival of women diagnosed with breast cancer in El-Minia governorate, Egypt. Int J Prev Med. 2011;2:131–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson C, Tobin S, Young R. The exploding worldwide cancer burden: The impact of cancer on women. Int J Gynecol Cancer. 2004;14:1–11. doi: 10.1111/j.1048-891x.2004.14178.x. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Vol. 2011. Atlanta: American Cancer Society; 2011. American Cancer Society (ACS). Cancer facts and figures 2011; pp. 9–10. [Google Scholar]
- 6.Mousavi SM, Montazeri A, Mohagheghi MA, Jarrahi AM, Harirchi I, Najafi M, et al. Breast cancer in Iran: An epidemiological review. Breast J. 2007;13:383–91. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 7.Emami Razavi S, Aghjani H, Haghazali M, Nadali F, Ramazani F, Dabiri E, et al. The most common cancers in Iranian women. Iran J Public Health. 2009;38:109–12. [Google Scholar]
- 8.Heydari Z, Mahmoudzadeh-Sagheb HR, Sakhavar N. Breast cancer screening knowledge and practice among women in southeast of Iran. Acta Med Iran. 2008;46:321–8. [Google Scholar]
- 9.Taleghani F, Khajehaminian MR, Khalifezadeh A, Karimian J, Hajahmadian H. The effect of exercise on physical aspect of quality of life in breast cancer patients undergoing chemotherapy. Iran J Nurs Midwifery Res. 2009;14:99–103. [Google Scholar]
- 10.Montazeri A, Ebrahimi M, Mehrdad N, Ansari M, Sajadian A. Delayed presentation in breast cancer: A study in Iranian women. BMC Womens Health. 2003;3:4. doi: 10.1186/1472-6874-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yavari P, Pourhoseingholi MA. Socioeconomic factors association with knowledge and practice of breast self-examination among Iranian women. Asian Pac J Cancer Prev. 2007;8:618–22. [PubMed] [Google Scholar]
- 12.Michell M. Breast cancer screening. Int J Clin Pract. 2001;55:531–5. [PubMed] [Google Scholar]
- 13.Bulletins ACoP. ACOG Practice Bulletin: Clinical management guidelines for obstetrician gynecologists. Number 122, August 2011: Breast Cancer screening (replaces practice bulletin, number 152, April 2003) Obstet Gynecol. 2011;122:1–11. [Google Scholar]
- 14.Mousavi SM, Mohaghegghi MA, Mousavi-Jerrahi A, Nahvijou A, Seddighi Z. Burden of breast cancer in Iran: A study of the Tehran population based cancer registry. Asian Pac J Cancer Prev. 2006;7:571–4. [PubMed] [Google Scholar]
- 15.Rahman S, Price JH, Dignan M, Lindquist PS, Jordan TR. Access to mammography facilities and detection of breast cancer by screening mammography: A gis approach. Int J Canc Prev. 2009;2:403–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney A, Murray M. Breast cancer screening recommendations: Is mammography the only answer? J Midwifery Womens Health. 2009;54:393–400. doi: 10.1016/j.jmwh.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Sandra A, Wendy S. 5th ed. Canada: Jones and Burtlett Publishers; 2009. Essential concepts for healthy living. [Google Scholar]
- 18.Mousavi SM, Harirchi I, Ebrahimi M, Mohagheghi MA, Montazeri A, Jarrahi AM, et al. Screening for breast cancer in Iran: a challenge for health policy makers. Breast J. 2008;14:605–6. doi: 10.1111/j.1524-4741.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 19.Dundar PE, Ozmen D, Ozturk B, Haspolat G, Akyildiz F, Coban S, et al. The knowledge and attitudes of breast self-examination and mammography in a group of women in a rural area in western Turkey. BMC Cancer. 2006;6:43. doi: 10.1186/1471-2407-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong A, Cheung P, Wong A, Hung G, Lo G, Tsao M, et al. The acceptance and feasibility of breast cancer screening in the East. Breast. 2008;17:42–50. doi: 10.1016/j.breast.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: A summary of the evidence for the U.S. Preventive services task force. Ann Intern Med. 2002;137(5 Part 1):347–60. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 22.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 23.Farshbaf Khalili A, Shahnazi M, Ghahvechi A, Thorabi SH. Performance conditions of breast cancer screening methods and its efficient factors among women referring to health centers of Tabriz. Iran J Nurs Res. 2009;4:27–38. [Google Scholar]
- 24.Salimi Pormehr SK, Sheykhan Z, Alavi Majd H. Investigation of breast cancer screening tests performance and affecting factors in women referred to Ardebil's health and medical centers. J Ardabil Univ Med Sci Health Serv. 2011;10:310–8. [Google Scholar]
- 25.Banaeian Sh KA, Kheiri S. Knowledge, attitude and practice about breast cancer screening and related factors among women referred to health care centers in Boroujen in 2005. Shahrekord Univ Med Sci J. 2006;7:34–28. [Google Scholar]
- 26.Hatefnia E, Niknami S, Bazargan M, Mahmoodi M, Lamyianm M, Alavi N. Correlates of mammography utilization among working Muslim Iranian women. Health Care Women Int. 2010;31:499–514. doi: 10.1080/07399331003725507. [DOI] [PubMed] [Google Scholar]
- 27.Abedian Kasgari KS, Yaghobi T. Health Believes of women about performing mammography among. Clients referred to health centers in Sari! Iran. MEJSR. 2011;7:683–8. [Google Scholar]
- 28.Secginli S, Nahcivan N. Factors associated with breast cancer screening behaviours in a sample of Turkish women: A questionnaire survey. Int J Nurs Stud. 2006;43:161–71. doi: 10.1016/j.ijnurstu.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Champion V, Maraj M, Hui S, Perkins AJ, Tierney W, Menon U, et al. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Prev Med. 2003;36:150–8. doi: 10.1016/s0091-7435(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 30.Wall KM, Nunez-Rocha GM, Salinas-Martinez AM, Sanchez-Pena SR. Determinants of the use of breast cancer screening among women workers in urban Mexico. Prev Chronic Dis. 2008;5:A50. [PMC free article] [PubMed] [Google Scholar]
- 31.Ho V, Yamal JM, Atkinson EN, Basen-Engquist K, Tortolero-Luna G, Follen M. Predictors of breast and cervical screening in Vietnamese women in Harris County, Houston, Texas. Cancer Nurs. 2005;28:119–29. doi: 10.1097/00002820-200503000-00005. quiz 30-1. [DOI] [PubMed] [Google Scholar]
- 32.Lamyian M, Hydarnia A, Ahmadi F, Faghihzadeh S, Aguilar-Vafaie M. Barriers to and factors facilitating breast cancer screening among Iranian women: A qualitative study. East Mediterr Health J. 2007;13:1160–70. doi: 10.26719/2007.13.5.1160. [DOI] [PubMed] [Google Scholar]
- 33.Boxwala FI, Bridgemohan A, Griffith DM, Soliman AS. Factors associated with breast cancer screening in Asian Indian women in metro-detroit. J Immigr Minor Health. 2010;12:534–43. doi: 10.1007/s10903-009-9277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer D, Reddick B, D’Agostino R, Jackson SA. Psychosocial correlates of mammography screening in older African American women. Oncol Nurs Forum. 2007;34:117–23. doi: 10.1188/07.ONF.117-123. [DOI] [PubMed] [Google Scholar]
- 35.Glanz K, Rimer BK, Viswanath K. USA, San Francisco: Jossey-Bass Inc Pub; 2008. Health behavior and health education: Theory, research, and practice; pp. 45–65. [Google Scholar]
- 36.Hayden J. USA: Jones and Bartlett Learning; 2009. Introduction to health behavior theory; pp. 31–7. [Google Scholar]
- 37.Gozum S, Karayurt O, Kav S, Platin N. Effectiveness of peer education for breast cancer screening and health beliefs in eastern Turkey. Cancer Nurs. 2010;33:213–20. doi: 10.1097/NCC.0b013e3181cb40a8. [DOI] [PubMed] [Google Scholar]
- 38.Avci IA, Kurt H. Health beliefs and mammography rates of Turkish women living in rural areas. J Nurs Scholarsh. 2008;40:170–5. doi: 10.1111/j.1547-5069.2008.00222.x. [DOI] [PubMed] [Google Scholar]
- 39.Canbulat N, Uzun Ö. Health beliefs and breast cancer screening behaviors among female health workers in Turkey1. Eur J Oncol Nurs. 2008;12:148–56. doi: 10.1016/j.ejon.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Champion VL, Monahan PO, Springston JK, Russell K, Zollinger TW, Saywell RM, Jr, et al. Measuring mammography and breast cancer beliefs in African American women. J Health Psychol. 2008;13:827–37. doi: 10.1177/1359105308093867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin ZC, Effken JA. Effects of a tailored web-based educational intervention on women's perceptions of and intentions to obtain mammography. J Clin Nurs. 2010;19:1261–9. doi: 10.1111/j.1365-2702.2009.03180.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosenstock IM. Why people use health services. Milbank Q. 2005;83:1–32. [Google Scholar]
- 43.Soskolne V, Marie S, Manor O. Beliefs, recommendations and intentions are important explanatory factors of mammography screening behavior among Muslim Arab women in Israel. Health Educ Res. 2007;22:665–76. doi: 10.1093/her/cyl132. [DOI] [PubMed] [Google Scholar]
- 44.Austin L, Ahmad F, McNally M, Stewart D. Breast and cervical cancer screening in Hispanic women: A literature review using the health belief model. Womens Health Issue. 2002;12:122–8. doi: 10.1016/s1049-3867(02)00132-9. [DOI] [PubMed] [Google Scholar]
- 45.Abbaszadeh A, Haghdoost A, Taebi M, Kohan S. The relationship between women's health beliefs and their participation in screening mammography. Asian Pac J Cancer Prev. 2007;8:471–5. [PubMed] [Google Scholar]
- 46.Champion VL, Scott CR. Reliability and validity of breast cancer screening belief scales in African American women. Nurs Res. 1997;46:331–7. doi: 10.1097/00006199-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Taymoori P, Berry T. The validity and reliability of Champion's Health Belief Model Scale for breast cancer screening behaviors among Iranian women. Cancer Nurs. 2009;32:465–72. doi: 10.1097/NCC.0b013e3181aaf124. [DOI] [PubMed] [Google Scholar]
- 48.Jarvandi S, Montazeri A, Harirchi I, Kazemnejad A. Beliefs and behaviours of Iranian teachers toward early detection of breast cancer and breast self-examination. Public Health. 2002;116:245–9. doi: 10.1038/sj.ph.1900854. [DOI] [PubMed] [Google Scholar]
- 49.Tejeda S, Thompson B, Coronado GD, Martin DP, Heagerty PJ. Predisposing and enabling factors associated with mammography use among Hispanic and non-Hispanic white women living in a rural area. J Rural Health. 2009 Winter;25:85–92. doi: 10.1111/j.1748-0361.2009.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tejeda S, Thompson B, Coronado GD, Martin DP. Barriers and facilitators related to mammography use among lower educated Mexican women in the USA. Soc Sci Med. 2009;68:832–9. doi: 10.1016/j.socscimed.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tariq M, Khubaib S, Imran A, Ibrahim M. Screening mammography for breast cancer in women using Bi-RADS scores. Iran J Cancer Prev. 2011:20–5. [Google Scholar]
- 52.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 53.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 54.Tabar L, Yen M, Vitak B, Chen H, Smith R, Duffy S. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–10. doi: 10.1016/S0140-6736(03)13143-1. [DOI] [PubMed] [Google Scholar]
- 55.Secginli S, Nahcivan NO. The effectiveness of a nurse-delivered breast health promotion program on breast cancer screening behaviours in non-adherent Turkish women: A randomized controlled trial. Int J Nurs Stud. 2011;48:24–36. doi: 10.1016/j.ijnurstu.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Fulton JP, Buechner JS, Scott HD, DeBuono BA, Feldman JP, Smith RA, et al. A study guided by the Health Belief Model of the predictors of breast cancer screening of women ages 40 and older. Public Health Rep. 1991;106:410–20. [PMC free article] [PubMed] [Google Scholar]
- 57.Savage SA, Clarke VA. Factors associated with screening mammography and breast self-examination intentions. Health Educ Res. 1996;11:409–21. doi: 10.1093/her/11.4.409-a. [DOI] [PubMed] [Google Scholar]
- 58.Gomez S, Tan S, Keegan T, Clarke C. Disparities in mammographic screening for Asian women in California: A cross-sectional analysis to identify meaningful groups for targeted intervention. BMC Cancer. 2007;7:201. doi: 10.1186/1471-2407-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bazargan M, Bazargan S, Calderon J, Husaini B, Baker R. Mammography screening and breast self-examination among minority women in public housing projects: The impact of physician recommendation. Cell Mol Biol (Noisy-le-Grand) 2003;49:1213–8. [PubMed] [Google Scholar]
- 60.Kerlikowske K, Smith-Bindman R, Ljung BM, Grady D. Evaluation of abnormal mammography results and palpable breast abnormalities. Ann Intern Med. 2003;139:274–84. doi: 10.7326/0003-4819-139-4-200308190-00010. [DOI] [PubMed] [Google Scholar]
- 61.Han Y, Williams RD, Harrison RA. Breast cancer screening knowledge, attitudes, and practices among Korean American women. Oncol Nurs Forum. 2000;27:1585–91. [PubMed] [Google Scholar]
- 62.Yu M, Wu T. Factors influencing mammography screening of Chinese American women. J Obstet Gynecol Neonatal Nurs. 2005;34:386–94. doi: 10.1177/0884217505276256. [DOI] [PubMed] [Google Scholar]
- 63.Yu X, McBean AM. Screening mammography use and chemotherapy among female stage II colon cancer patients: A retrospective cohort study. BMC Health Serv Res. 2010;10:98. doi: 10.1186/1472-6963-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15:175–83. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 65.Ham OK. Factors affecting mammography behavior and intention among Korean women. Oncol Nurs Forum. 2006;33:113–9. doi: 10.1188/06.ONF.113-119. [DOI] [PubMed] [Google Scholar]
- 66.Petroc-Nustas WI. Factors associated with mammography utilization among Jordanian women. J Transcult Nurs. 2001;12:284–91. doi: 10.1177/104365960101200403. [DOI] [PubMed] [Google Scholar]
- 67.Magai C, Consedine N, Conway F, Neugut A, Culver C. Diversity matters: Unique populations of women and breast cancer screening. Cancer. 2004;100:2300–7. doi: 10.1002/cncr.20278. [DOI] [PubMed] [Google Scholar]
- 68.Wu TY, West B, Chen YW, Hergert C. Health beliefs and practices related to breast cancer screening in Filipino, Chinese and Asian-Indian women. Cancer Detect Prev. 2006;30:58–66. doi: 10.1016/j.cdp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Haghighat SH, Ansari M, Yunesian M, Akbari ME, Ebrahimi M, Alijani M, et al. Telephone and face-to-face consultation in breast cancer diagnosis: A comparative study. Iran J Breast Dis. 2009;2:7–12. [Google Scholar]
- 70.Smith EM. Telephone interviewing in healthcare research: A summary of the evidence. Nurse Res. 2005;12:32–41. doi: 10.7748/nr2005.01.12.3.32.c5946. [DOI] [PubMed] [Google Scholar]
- 71.Cohen L, Manion L, Morrison K, Morrison K, Publishers R. Book Reviews:Research methods in education (6th ed). Oxford, UK. The Australian Educational Researcher. 2009;36:147–56. [Google Scholar]
- 72.Sturges JE, Hanrahan KJ. Comparing telephone and face-to-face qualitative interviewing: A research note. Qual Res. 2004;4:107–18. [Google Scholar]
- 73.Musselwhite K, Cuff L, McGregor L, King KM. The telephone interview is an effective method of data collection in clinical nursing research: A discussion paper. Int J Nurs Stud. 2007;44:1064–70. doi: 10.1016/j.ijnurstu.2006.05.014. [DOI] [PubMed] [Google Scholar]


