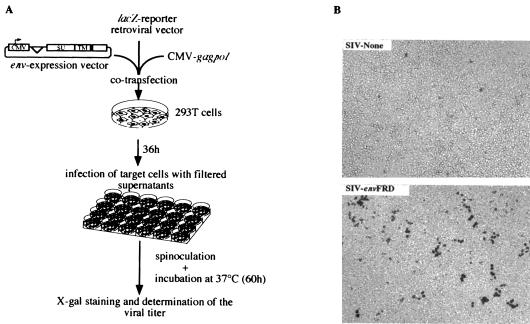
FIG. 1.
Infection assays using retroviral particles pseudotyped with the HERV-FRD and control envelopes. (A) Rationale of the assay. To determine whether the HERV-FRD envelope could confer infectivity on MLV, HIV, or SIV Env-defective pseudoparticles, corresponding pseudotype viruses were produced by cotransfecting 7.5 × 105 293T cells with 0.55 μg of the phCMV-HERV-FRD vector expressing the HERV-FRD envelope (or pcDNA3 for the negative control and phCMVampho for the amphotropic MLV envelope positive control); 1.75 μg of a vector encoding the retroviral proteins (except the envelope) of MLV (phCMVintron) (13), HIV (pCMVΔR8.91) (20), or SIV (pSIV3+) (13); and 1.75 μg of the corresponding defective retroviral vectors marked with a β-galactosidase reporter gene (pMFGsnlslacZ [7], pHR′CMVlacZ [20], and R9SA [14]), by calcium phosphate precipitation (Invitrogen). At 36 h posttransfection, viral supernatants were collected and filtered through 0.45-μm-pore-size membranes. Target cells (murine 3T3; feline G355-5; or human TE671, HeLa, and 293T cells) were seeded in 24-well plates at a density of 104 cells per well and incubated overnight at 37°C. Five to 500 μl of virus samples containing 4 μg of Polybrene per ml was added to the cells and centrifuged for spinoculation at 1,200 × g for 2 h 30 min at 25°C (10). After removal of the supernatants, the cells were incubated in regular medium for 60 h at 37°C. Viral titers were then measured by X-Gal (5-bromo-4-chloro-3-indolylphosphate) staining of the cells and expressed as lacZ CFU per milliliter of viral supernatant. (B) Infectivity of SIV-based retroviral particles pseudotyped with the HERV-FRD envelope (or with no protein in the “SIV-None” control), as assayed using human 293T cells as target cells and X-Gal staining, following the protocol described for panel A.