Abstract
We and others have presented evidence for a direct interaction between the matrix (MA) domain of the human immunodeficiency virus type 1 (HIV-1) Gag protein and the cytoplasmic tail of the transmembrane envelope (Env) glycoprotein gp41. In addition, it has been postulated that the MA domain of Gag undergoes a conformational change following Gag processing, and the cytoplasmic tail of gp41 has been shown to modulate Env-mediated membrane fusion activity. Together, these results raise the possibility that the interaction between the gp41 cytoplasmic tail and MA is regulated by protease (PR)-mediated Gag processing, perhaps affecting Env function. To examine whether Gag processing affects Env-mediated fusion, we compared the ability of wild-type (WT) HIV-1 Env and a mutant lacking the gp41 cytoplasmic tail to induce fusion in the context of an active (PR+) or inactive (PR−) viral PR. We observed that PR− virions bearing WT Env displayed defects in cell-cell fusion. Impaired fusion did not appear to be due to differences in the levels of virion-associated Env, in CD4-dependent binding to target cells, or in the formation of the CD4-induced gp41 six-helix bundle. Interestingly, truncation of the gp41 cytoplasmic tail reversed the fusion defect. These results suggest that interactions between unprocessed Gag and the gp41 cytoplasmic tail suppress fusion.
During or shortly after virus release from the plasma membrane of the infected cell, the human immunodeficiency virus type 1 (HIV-1) protease (PR) cleaves the Gag and Gag-Pol polyprotein precursors to generate the mature Gag and Pol proteins. This PR-mediated processing of Gag and Gag-Pol precursors leads to a striking transformation in virion morphology, a process known as virus maturation. During maturation, noninfectious particles with electron-lucent cores are converted to infectious virions with electron-dense, conical cores (50, 51, 55). It has been postulated that Gag processing induces conformational changes in the matrix (MA) domain of Gag (25, 44, 59). Although it has long been appreciated that immature virions are noninfectious (24, 32, 41), the nature of the infectivity block and the step in virus entry that is affected remain to be determined.
The HIV-1 Env glycoproteins are synthesized as a 160-kDa precursor protein, gp160, which is cleaved by cellular proteases during trafficking to the plasma membrane to generate the mature surface glycoprotein gp120 and the transmembrane glycoprotein gp41. The gp120/gp41 Env glycoprotein complex is incorporated into virus particles during the assembly process. On the mature HIV-1 virion, gp120 and gp41 act in concert to catalyze the fusion of viral and target cell membranes, resulting in the delivery of the viral core into the cytoplasm. Env-mediated fusion takes place in a series of steps: binding of gp120 to the HIV-1 receptor CD4, interaction between gp120 and a coreceptor (typically CXCR4 or CCR5), formation of a gp41 ectodomain six-helix bundle (6HB), hemifusion, and fusion pore formation (2, 16, 21).
A number of studies have provided evidence for a functional interaction between the long cytoplasmic tail (CT) of gp41 and the MA domain of Gag. (i) Deletions (57) and point mutations (22) in MA block Env incorporation into virus particles. (ii) Truncation of the gp41 CT reverses the Env incorporation defect imposed by MA mutations (20, 22, 37). (iii) An Env incorporation defect resulting from a small deletion near the middle of the gp41 CT is reversed by a specific point mutation in MA (38). In addition, we and others found that viral cores prepared from PR− virions contained high levels of Pr55Gag and gp41 (58; T. Murakami and E. O. Freed, unpublished data). Interestingly, this detergent-resistant interaction between gp41 and Pr55Gag is dependent on the gp41 CT, again suggesting that the CT is required for the Gag-gp41 interaction. Furthermore, several lines of evidence suggest a connection between the CT of retroviral Env glycoproteins and membrane fusion. For example, in the case of certain retroviruses (e.g., murine leukemia virus and Mason-Pfizer monkey virus), the Env CT is cleaved by the viral PR after virus release, and this cleavage event activates Env fusogenicity (5, 42, 43). In addition, truncation of the CT of the maedi-visna virus (58), simian immunodeficiency virus (SIV) (45, 49, 60), HIV-1 (13, 15, 20, 54), and human T-cell leukemia virus type 1 (31) transmembrane glycoproteins increases fusion activity. Together, these observations raise the possibility that interactions between HIV-1 Gag and the gp41 CT may affect Env-mediated fusion and that fusion could thus be influenced by the state of Gag processing.
In this study, we investigated whether Gag processing and virion maturation affect HIV-1 Env-mediated membrane fusion. We found that inactivating HIV-1 PR suppresses the ability of HIV-1 virions to induce cell-to-cell fusion, a type of fusion known as fusion from without (3, 11, 28). The fusion defect is not the result of differences in gp120/gp41 levels on PR+ versus PR− virions and appears to be imposed at a step following CD4 binding and receptor-activated conformational changes in gp41. Interestingly, the fusion defect is reversed by truncating the CT of gp41, suggesting that interactions between unprocessed Gag and the gp41 CT suppress fusion.
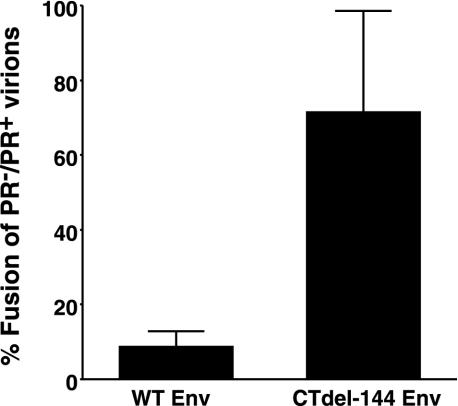
PR− virions bearing WT Env display defects in cell-cell fusion assays.
To examine whether Gag processing affects HIV-1 Env-mediated fusion, we compared the ability of HIV-1 virions to induce fusion from without (11) in the context of either active (PR+) or inactive (PR−) viral PR (27). To prevent productive infection and thereby ensure that our assays accurately measure fusion from without, we used pseudovirions produced by cotransfecting an Env-defective HIV-1 NL4-3 molecular clone (NL4-3/KFS) (19, 22) or its PR− counterpart (NL4-3/PR−/KFS) with a wild-type (WT) NL4-3 Env expression vector. Concentrated pseudovirions were added to a Jurkat T-cell line, and the extent of fusion was determined by scoring syncytia (whose diameters were more than four times those of unfused Jurkat cells). PR+ virions efficiently induced fusion; in contrast, fusion induced by PR− virions was reduced by 90% compared with levels observed with PR+ virions (Fig. 1). We postulated that if the fusion defect observed with PR− virions was a consequence of interactions between unprocessed Gag and the gp41 CT, then the suppressive effect on fusion would be reversed by removing the CT. To test this hypothesis, we prepared PR+ and PR− pseudovirions bearing an Env mutant (CTdel-144) lacking the CT. This mutant was constructed by introducing two adjacent stop codons in the env gene, such that a truncated form of gp41 lacking the C-terminal 144 amino acids was expressed (39). Intriguingly, and consistent with our hypothesis, pseudovirions bearing the CTdel-144 Env induced fusion in both PR+ and PR− contexts (Fig. 1). We obtained results essentially identical to those presented in Fig. 1 by using a highly quantitative fusion assay in which Tat-expressing Jurkat (i.e., Jurkat-tat) cells (6) and LuSIV cells containing a luciferase reporter gene under the control of the SIV long terminal repeat (46) were cocultivated in the presence of concentrated pseudovirions (data not shown). These results support the hypothesis that Gag processing activates virus-induced cell-cell fusion mediated by the WT HIV-1 Env.
FIG. 1.
PR− virions display a fusion defect in a cell-cell fusion-from-without assay. env-defective HIV-1 NL4-3 (NL4-3/KFS) (19, 22) and its PR− counterpart were cotransfected into 293T cells with pUC19, with vectors expressing the WT NL4-3 Env (pIIINL4env) (39) or the CT truncation mutant CTdel-144 (pNL4envCTdel-144) (39). Virus-containing supernatants were harvested 2 days posttransfection, and virions were concentrated (10 to 20×) by centrifugation (20,000 × g for 2 h). The concentrated pseudovirions were added to Jurkat cells. After a 20-h cultivation, the number of syncytia (whose diameters were more than four times those of unfused Jurkat cells) was scored. The number of syncytia was expressed as a ratio (%) of those obtained with PR− versus PR+ virions. The average number of syncytia induced by PR+ virions bearing WT and CTdel-144 Env was 171 and 116, respectively. Data are means of the results of four independent experiments. Error bars indicate standard deviations.
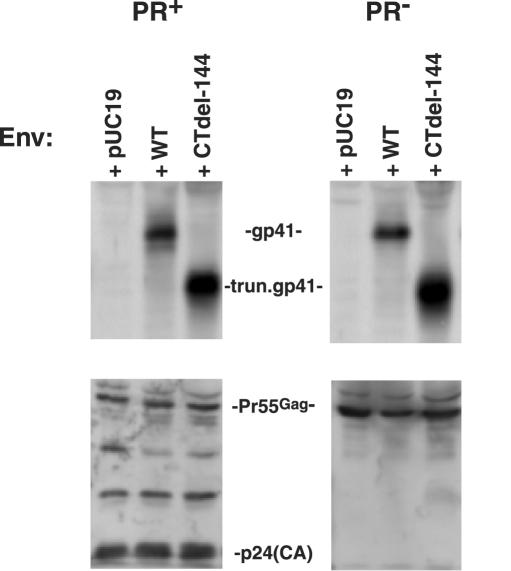
Differences in the ability of PR+ versus PR− virions to induce fusion are not attributable to different levels of virion-associated Env.
To explore the possibility that PR− virions are fusion defective due to reduced levels of virion-associated Env, we measured levels of gp41 on PR+ and PR− virions by Western blotting. Amounts of virion-associated gp41 were found to be unaffected by whether the virions were PR+ or PR−; this result was observed for both the WT Env and the CTdel-144 truncation mutant (Fig. 2). We also determined by radioimmunoprecipitation analysis that the state of Gag processing did not affect gp120 levels on virions bearing either WT or CTdel-144 Env (data not shown). Thus, differences in the ability of PR+ and PR− virions to induce fusion from without do not result from different levels of virion-associated Env.
FIG. 2.
PR+ and PR− virions incorporate comparable levels of gp41. Virion lysates, prepared from concentrated virus stocks as described in the legend of Fig. 1, were transferred to polyvinylidene difluoride membranes and immunoblotted with the anti-gp41 MAb T32 (upper panel) and AIDS patient serum (lower panel) to detect p24 (capsid) and/or Pr55Gag. To confirm equal loading of virion-associated material, blots were reprobed with an anti-Vpr antibody (data not shown). Quantitative Western blotting was performed with a Fluor-S MAX MultiImager (Bio-Rad, Hercules, Calif.). T32 was obtained from P. Earl (14), and AIDS patient serum was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. The data shown are representative of the results of at least five independent experiments.
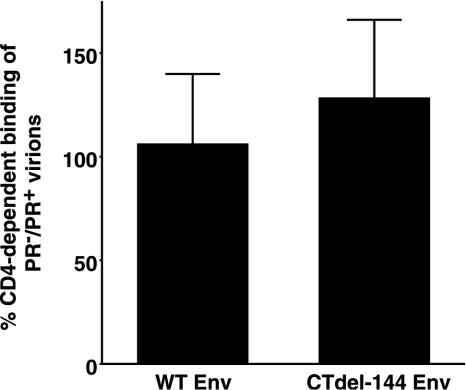
The fusion defect displayed by PR− virions is not due to impaired CD4-dependent cell surface binding or major effects on 6HB formation.
To investigate which step in virus entry is blocked in the absence of Gag processing, we examined whether Gag processing affects CD4-dependent HIV-1 binding to target T cells. For this purpose, we selected the Molt-4 T-cell line (30), which has been reported to contain low levels of heparan sulfate (40) and therefore displays limited CD4-independent virion binding. Molt-4 cells were incubated (37°C for 30 min) with concentrated HIV-1 pseudovirions in the presence of anti-CD4 monoclonal antibody (MAb) (Beckman 13B8.2) or control immunoglobulin G1 (IgG1). The virus-bound cells were stained with a rat anti-gp120 MAb (W#10) recognizing RIQRGPG (Y. Tanaka, unpublished data), and the amount of bound virus was determined by flow cytometry. Pseudovirions bearing WT NL4-3 Env showed comparable levels of CD4-dependent binding (about 95% of total binding) to Molt-4 cells in either the PR+ or PR− context. Pseudovirions bearing CTdel-144 Env displayed a slight, statistically insignificant increase (P = 0.19, t test) in binding in the PR− context (Fig. 3). These results indicate that the fusion defect displayed by PR− virions is not due to differences in CD4-dependent binding to the surface of target cells.
FIG. 3.
PR+ and PR− virions display comparable levels of CD4-dependent cell surface binding. Molt-4 clone 8 cells (5 × 105) (30) were incubated with concentrated HIV-1 pseudovirions (prepared as described in the legend of Fig. 1) in the presence of anti-CD4 MAb (Beckman, 13B8.2) or control IgG1. Virus-bound cells were stained with a rat anti-gp120 MAb (W#10) followed by the addition of phycoerythrin-conjugated goat anti-rat IgG. The amount of virus bound was determined by flow cytometry. CD4-independent binding determined by measuring the amount of virus bound in the presence of the anti-CD4 MAb was subtracted from the total binding. Data are means ± standard deviations of the results of four independent experiments.
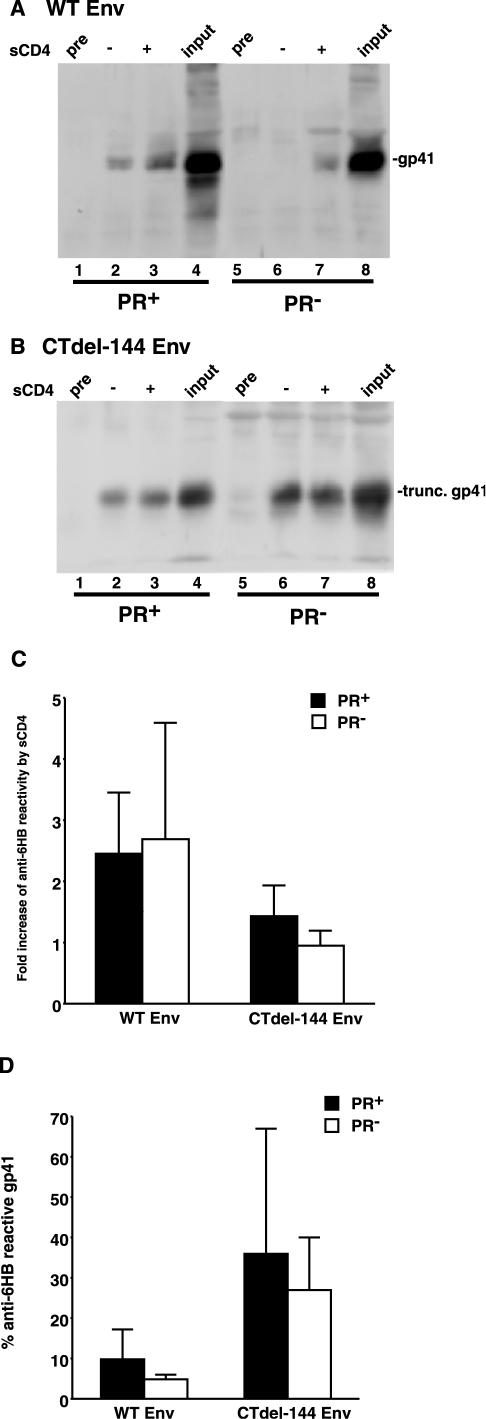
It has been demonstrated that Env-CD4 binding triggers a series of conformational changes in gp120 and gp41 that ultimately lead to the formation of a 6HB in the ectodomain of gp41 (16). To investigate the possibility that receptor-activated formation of the 6HB is blocked in the absence of Gag processing, immunoprecipitation assays were performed using intact HIV-1 virions incubated with an anti-6HB antiserum (rabbit serum no. 948, kindly provided by C. Weiss, U.S. Food and Drug Administration) (12, 23) or control preimmune serum in the presence or absence of soluble CD4 (sCD4) (Fig. 4). The immunoprecipitated material was then subjected to Western blotting with the anti-gp41 MAb T32 (kindly provided by P. Earl, National Institute of Allergy and Infectious Diseases) (14). As previously reported (12), sCD4, by using intact Env-expressing cells, enhanced (by 2.5-fold) immunoprecipitation of gp41 by the anti-6HB Ab (Fig. 4A, lanes 2 and 3, and Fig. 4C). An enhanced (2.7-fold) immunoprecipitation was also induced by sCD4 in PR− virions bearing WT NL4-3 Env (Fig. 4A, lanes 6 and 7, and Fig. 4C). For CTdel-144, reactivity to the anti-6HB Ab was enhanced slightly (1.4-fold) by sCD4 in the PR+ context (Fig. 4B, lanes 2 and 3, and Fig. 4C), whereas there was no substantial enhancement by sCD4 in PR− virions (Fig. 4B, lanes 6 and 7, and Fig. 4C). We also assessed the extent of 6HB formation by calculating the fraction of virion-associated gp41 detected by the anti-6HB Ab following sCD4 treatment versus total virion-associated gp41 (Fig. 4D). Again, the CTdel-144 mutant showed an increase in 6HB formation relative to WT. Reproducible but statistically insignificant reductions in 6HB formation were seen for both WT and CTdel-144 Env in the PR− context. Together, these results suggest that the fusion defect displayed by PR− virions is not due to a block in 6HB formation. However, at this time we cannot exclude the possibility that the anti-6HB immunoprecipitation approach used here might not detect small but meaningful differences in formation of the 6HB.
FIG.4.
PR+ and PR− virions display comparable levels of 6HB formation. Concentrated HIV-1 virions (prepared as described in the legend of Fig. 1, except that in this case NL4-3 [1] and CTdel-144 [39] and their PR− counterparts were used) were incubated with an anti-6HB antiserum (rabbit serum no. 948) or preimmune serum (pre) in the presence or absence of 1 μg of sCD4 (Immuno Diagnostics) at 37°C for 2 h. Virions were then pelleted by centrifugation (20,000 × g for 2 h) prior to lysis and immunoprecipitation with protein G Sepharose beads. The immunoprecipitated material was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to quantitative Western blotting with the anti-gp41 MAb T32. An amount of viral lysate equivalent to input virus was run as a positive control. Gels were run for WT Env (A) and CTdel-144 Env (B). The increase in anti-6HB reactivity (n-fold) induced by sCD4 in the PR+ (solid bar) and PR− (open bar) context (C) is shown. Also shown is the percentage of anti-6HB-reactive gp41, calculated by dividing the amount of 6HB-reactive gp41 following sCD4 addition by input gp41 in PR+ (solid bar) and PR− (open bar) virions (D). Rabbit serum no. 948 was obtained from C. Weiss (12, 23). Data are means ± standard deviations of the results of at least three independent experiments.
Conclusions.
In the present study, we hypothesized that the MA-gp41 CT interaction may be altered by Gag processing, perhaps affecting Env function. Indeed, we found that inactivating HIV-1 PR suppresses Env-mediated cell-cell fusion from without. The fact that the fusion defect is rescued by truncating the gp41 CT suggests that interactions between unprocessed Gag and the gp41 CT inhibit fusion. These results demonstrate a heretofore unappreciated role of the viral PR in activating infectivity and suggest that a fusion defect imposed by unprocessed Gag may contribute to the high degree of potency displayed by PR inhibitors.
How might MA-gp41 interactions suppress membrane fusion? We and others have observed a detergent-resistant association between Pr55Gag and gp41 in immature (PR−) HIV-1 virions (56; Murakami and Freed, unpublished). This detergent-resistant Gag-gp41 interaction is not detected in mature (PR+) particles. We speculate that a physical association between Gag and the gp41 CT locks the Env glycoprotein complex into a nonfusogenic conformation that is reversed by PR-mediated Gag processing or by gp41 mutations that eliminate the Gag-gp41 interaction. Interestingly, previous reports suggested that the association between matrix proteins and the CT of envelope proteins may negatively regulate the fusogenicity of measles and Newcastle disease viruses (7, 8, 47). Taken together, it can be postulated that a variety of enveloped viruses have evolved mechanisms, including MA-Env interactions and PR-mediated Env CT cleavage (5, 42, 43), to suppress Env-induced membrane fusion until virus budding has been completed. Such mechanisms would limit fusion-induced cytopathicity and abortive reinfections.
To define the step in the fusion process inhibited by unprocessed Gag, we first determined that the state of the viral PR does not affect levels of virion-associated Env. This conclusion is in agreement with that of a previous biochemical report (35) and with morphological and structural analyses of both mature and immature HIV-1 virions by cryoelectron microscopy, which revealed a comparable density of Env proteins on both types of virions (4, 53). Next, we examined whether Gag processing affects virus binding to target CD4+ T cells. We observed that Env proteins on PR− virions are functionally competent in the attachment step. Finally, we tested whether 6HB formation takes place to a comparable extent on PR+ versus PR− virions. 6HB formation occurs during membrane fusion induced by a number of different viral glycoproteins, including those of HIV-1 (16, 48), and several lines of evidence suggest that this structure directly participates in fusion (29, 36, 52). Our results suggest that the enhanced immunoprecipitation by anti-6HB antibodies induced by sCD4 treatment in virions bearing WT NL4-3 Env is not affected by the state of Gag processing, though the inherent variability in the detection assay would make subtle differences difficult to measure. Interestingly, relative to WT Env, CTdel-144 mutant Env appears to be highly reactive with the anti-6HB Ab even without sCD4 treatment. This phenomenon may in part explain why CD4-independent isolates often acquire CT truncations (17, 26, 34). Indeed, Edwards et al. recently reported that truncation of the gp41 CT enhances not only binding of monoclonal antibodies to CD4-induced epitopes in the ectodomain of gp120 but also neutralization sensitivity to HIV-1-positive serum (18). Thus, our results suggest that the fusion defect observed with PR− virions is elicited at a step following 6HB formation, e.g., hemifusion or the initiation and enlargement of the fusion pore. Gag processing may be necessary for the assembly of the higher-order Env oligomers that are postulated to be required for fusion (9, 19, 33). Given the low level of gp120 and gp41 on the surface of HIV-1 virions (10), Env trimers must presumably be free to diffuse and cluster in the plane of the lipid bilayer in order to form fusion-active, higher-order complexes. Interactions between the gp41 CT and unprocessed Gag may limit the ability of Env trimers to diffuse in the plane of the lipid bilayer, thereby inhibiting fusion pore formation. It is also possible that PR-mediated Gag processing and concomitant reorganization of the structure of the mature virion may affect the interaction of Env with lipids or other components of the viral (or cell) membrane, thereby in some manner activating membrane fusion. Alternatively, one of the mature Gag proteins could directly or indirectly influence the fusion reaction.
In summary, we describe here a fundamentally novel observation regarding the functional relationship between the HIV-1 Gag and Env proteins. Work is ongoing in our laboratories to further define the ability of Gag to regulate Env function and the implications of this regulation to our understanding of HIV-1 replication and the development of antiviral agents.
Acknowledgments
We thank A. Ono and R. Goila-Gaur for critical reviews of the manuscript. The following reagents were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Jurkat-tat cells were from A. Caputo, W. A. Haseltine, and J. G. Sodroski; LuSIV cells were from J. W. Roos and J. E. Clements; and AIDS patient serum was from L. Vujcic. We thank A. Engleman for the RT− mutant and P. Earl and C. Weiss for antibodies.
This work was supported by a grant-in-aid for scientific research (C) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 3.Bratt, M. A., and W. R. Gallaher. 1969. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc. Natl. Acad. Sci. USA 64:536-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs, J. A., T. Wilk, R. Welker, H. G. Kräusslich, and S. D. Fuller. 2003. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 22:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody, B. A., S. S. Rhee, and E. Hunter. 1994. Postassembly cleavage of a retroviral glycoprotein cytoplasmic domain removes a necessary incorporation signal and activates fusion activity. J. Virol. 68:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caputo, A., J. G. Sodroski, and W. A. Haseltine. 1990. Constitutive expression of HIV-1 tat protein in human Jurkat T cells using a BK virus vector. J. Acquir. Immune Defic. Syndr. 3:372-379. [PubMed] [Google Scholar]
- 7.Cathomen, T., B. Mrkic, D. Spehner, R. Drillien, R. Naef, J. Pavlovic, A. Aguzzi, M. A. Billeter, and R. Cattaneo. 1998. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 17:3899-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 10.Chertova, E., J. W. Bess, Jr., B. J. Crise, I. R. Sowder, T. M. Schaden, J. M. Hilburn, J. A. Hoxie, R. E. Benveniste, J. D. Lifson, L. E. Henderson, and L. O. Arthur. 2002. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 76:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavel, F., and P. Charneau. 1994. Fusion from without directed by human immunodeficiency virus particles. J. Virol. 68:1179-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Rosny, E., R. Vassell, P. T. Wingfield, C. T. Wild, and C. D. Weiss. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J. Virol. 75:8859-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards, T. G., S. Wyss, J. D. Reeves, S. Zolla-Pazner, J. A. Hoxie, R. W. Doms, and F. Baribaud. 2002. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J. Virol. 76:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freed, E. O., E. L. Delwart, G. L. Buchschacher, Jr., and A. T. Panganiban. 1992. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc. Natl. Acad. Sci. USA 89:70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freed, E. O., and M. A. Martin. 2001. HIVs and their replication, p. 1971-2041. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 22.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Göttlinger, H. G., J. G. Sodroski, and W. A. Haseltine. 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:5781-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermida-Matsumoto, L., and M. D. Resh. 1999. Human immunodeficiency virus type 1 protease triggers a myristoyl switch that modulates membrane binding of Pr55gag and p17MA. J. Virol. 73:1902-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman, T. L., C. C. LaBranche, W. Zhang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 96:6359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iorio, R. M., and R. L. Glickman. 1992. Fusion mutants of Newcastle disease virus selected with monoclonal antibodies to the hemagglutinin-neuraminidase. J. Virol. 66:6626-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji, H., W. Shu, F. T. Burling, S. Jiang, and M. Lu. 1999. Inhibition of human immunodeficiency virus type 1 infectivity by the gp41 core: role of a conserved hydrophobic cavity in membrane fusion. J. Virol. 73:8578-8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikukawa, R., Y. Koyanagi, S. Harada, N. Kobayashi, M. Hatanaka, and N. Yamamoto. 1986. Differential susceptibility to the acquired immunodeficiency syndrome retrovirus in cloned cells of human leukemic T-cell line Molt-4. J. Virol. 57:1159-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, F. J., N. Manel, Y. Boublik, J. L. Battini, and M. Sitbon. 2003. Human T-cell leukemia virus type 1 envelope-mediated syncytium formation can be activated in resistant mammalian cell lines by a carboxy-terminal truncation of the envelope cytoplasmic domain. J. Virol. 77:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl, N. E., E. A. Emini, W. A. Schleif, L. J. Davis, J. C. Heimbach, R. A. Dixon, E. M. Scolnick, and I. S. Sigal. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA 85:4686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaBranche, C. C., T. L. Hoffman, J. Romano, B. S. Haggarty, T. G. Edwards, T. J. Matthews, R. W. Doms, and J. A. Hoxie. 1999. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J. Virol. 73:10310-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert, D. M., S. R. Petteway, Jr., C. E. McDanal, T. K. Hart, J. J. Leary, G. B. Dreyer, T. D. Meek, P. J. Bugelski, D. P. Bolognesi, B. W. Metcalf, and T. M. Matthews. 1992. Human immunodeficiency virus type 1 protease inhibitors irreversibly block infectivity of purified virions from chronically infected cells. Antimicrob. Agents Chemother. 36:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, M., M. O. Stoller, S. Wang, J. Liu, M. B. Fagan, and J. H. Nunberg. 2001. Structural and functional analysis of interhelical interactions in the human immunodeficiency virus type 1 gp41 envelope glycoprotein by alanine-scanning mutagenesis. J. Virol. 75:11146-11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Göttlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami, T., and E. O. Freed. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and α-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohshiro, Y., T. Murakami, K. Matsuda, K. Nishioka, K. Yoshida, and N. Yamamoto. 1996. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol. Immunol. 40:827-835. [DOI] [PubMed] [Google Scholar]
- 41.Peng, C., B. K. Ho, T. W. Chang, and N. T. Chang. 1989. Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J. Virol. 63:2550-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragheb, J. A., and W. F. Anderson. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451:1-16. [DOI] [PubMed] [Google Scholar]
- 45.Ritter, G. D., Jr., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 46.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 47.Russell, P. H. 1984. Newcastle disease virus: the effect of monoclonal antibody in the overlay on virus penetration and the immunoselection of variants. J. Gen. Virol. 65:795-798. [DOI] [PubMed] [Google Scholar]
- 48.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 49.Spies, C. P., and R. W. Compans. 1994. Effects of cytoplasmic domain length on cell surface expression and syncytium-forming capacity of the simian immunodeficiency virus envelope glycoprotein. Virology 203:8-19. [DOI] [PubMed] [Google Scholar]
- 50.Swanstrom, R., and J. W. Wills. 1997. Synthesis, assembly, and processing of viral proteins, p. 263-334. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 51.Vogt, V. M. 1997. Retroviral virions and genomes, p. 27-69. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 52.Weng, Y., Z. Yang, and C. D. Weiss. 2000. Structure-function studies of the self-assembly domain of the human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 74:5368-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilk, T., I. Gross, B. E. Gowen, T. Rutten, F. de Haas, R. Welker, H. G. Kräusslich, P. Boulanger, and S. D. Fuller. 2001. Organization of immature human immunodeficiency virus type 1. J. Virol. 75:759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 55.Wills, J. W., and R. C. Craven. 1991. Form, function, and use of retroviral gag proteins. AIDS 5:639-654. [DOI] [PubMed] [Google Scholar]
- 56.Wyma, D. J., A. Kotov, and C. Aiken. 2000. Evidence for a stable interaction of gp41 with Pr55Gag in immature human immunodeficiency virus type 1 particles. J. Virol. 74:9381-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu, X., X. Yuan, Z. Matsuda, T. H. Lee, and M. Essex. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeilfelder, U., and V. Bosch. 2001. Properties of wild-type, C-terminally truncated, and chimeric maedi-visna virus glycoprotein and putative pseudotyping of retroviral vector particles. J. Virol. 75:548-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, W., and M. D. Resh. 1996. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 70:8540-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases Env incorporation into particles and fusogenicity and infectivity. J. Virol. 67:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]