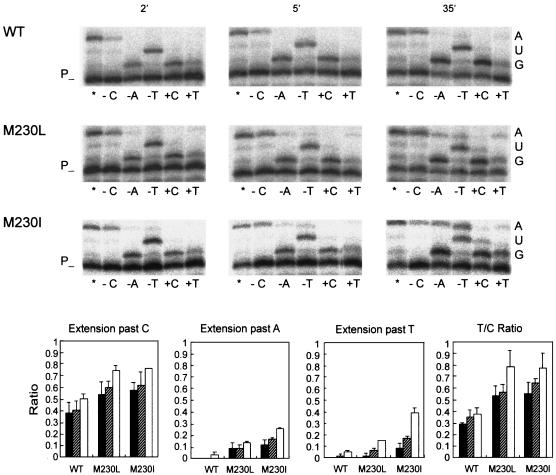
FIG. 2.
Extension of primer 2TRP by WT and mutant RTs in assays containing an RNA template. The nucleotides used in these assays were dATP-dCTP-dTTP (lane *), dATP-dTTP (lane −C), dCTP-dTTP (lane −A), dATP-dCTP (lane −T), dCTP alone (lane +C), and dTTP alone (lane +T). Template RNA nucleotides at positions +1, +2 and +3 are shown on the right and include the first 2 nt of the second Trp codon in the insert analyzed. The labeled template-primer at 30 nM was incubated with 20 to 40 nM RT at 37°C for up to 35 min in a total volume of 24 μl containing 50 mM HEPES (pH 7.0), 15 mM NaCl, 15 mM magnesium acetate, 130 mM KCH3COO, 1 mM DTT, and 5% polyethylene glycol 6000 (23). The concentration of all nucleotides in the extension reaction mixtures was 5 μM. Histograms below show the relative fidelities of the WT and mutant RTs. The amount of extension products was quantitated by phosphorimaging. Maximum extensions were determined for each enzyme and time point, as the sum of the intensities of bands at positions +1, +2 and +3, relative to the intensity of the band corresponding to the unextended primer, in reactions carried out with 3 nt (lane *). In the absence of dCTP (lane −C), dATP (lane −A), or dTTP (lane −T), misinsertion and mispair extension errors render bands at position +3 (i.e., in reactions shown in lanes −A and −T) or bands at positions +2 and +3 (lane −C). Reported ratios were obtained from the intensities of these bands referred to those obtained for unextended or shorter primers and then divided by the maximum intensity as obtained from results in lane *. The T/C ratio was determined as the relative amount of extended primer in the presence of dTTP (lane +T) compared with that in the presence of dCTP (lane +C) for each enzyme and time point. All results represent the average ± the standard deviation from three independent experiments.