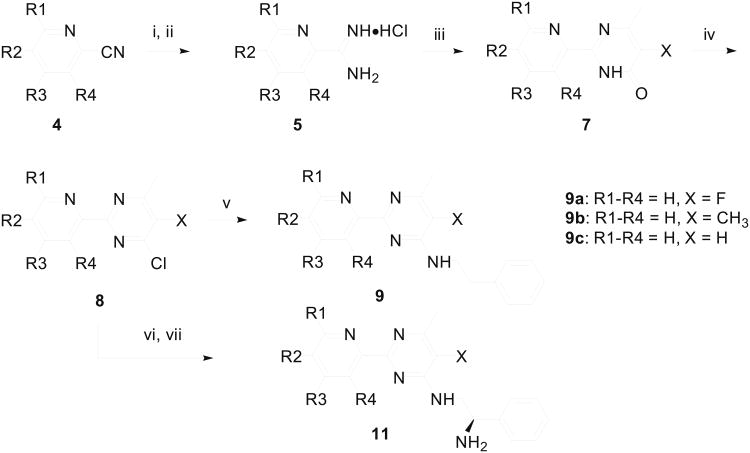
Scheme 1.
Synthesis of 2-pyridinyl-6-methylpyrimidines. Reagents & Conditions: (i) MeONa/MeOH, rt; (ii) NH4Cl/MeOH, 65-74% yields for two steps; (iii) H3C-COCH(X)CO2Et (6), MeONa/MeOH, 45-53% yields; (iv) POCl3, reflux, 65-72% yields; (v) PhCH2CH2NH2, K2CO3/MeOH, 65 °C, 85-92% yield; (vi) (R)-H2NCH2CH(Ph)NHBoc (10), Et3N, THF, 82-95% yields; (vii) CF3CO2H/CH2Cl2, then aqueous NaHCO3, 81-86% yields. Yield for 9a, 9b, and 9c were 56%, 62%, and 49% over three steps, respectively.