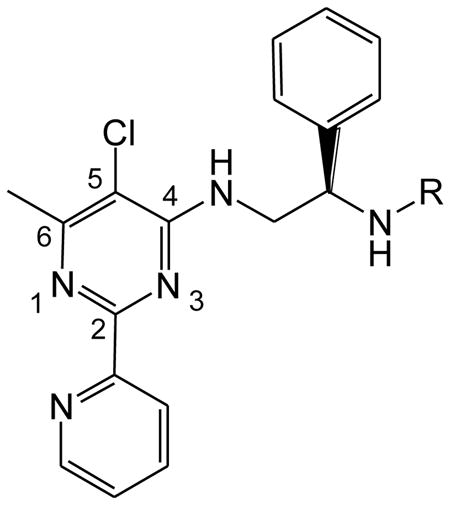
Table 2. Inhibition of purified recombinant human MetAPs by pyridinylpyrimidine derivatives.
| ||||
|---|---|---|---|---|
|
| ||||
| Cmpd | R | IC50a (μM) | IC50 ratio (type 2/1) | |
|
| ||||
| HsMetAP1 | HsMetAP2 | |||
| 16a |
|
0.8 ± 0.1 | 114 ± 12 | 143 |
| 16b |
|
1.1 ± 0.1 | >300 | >272 |
| 18a |
|
0.6 ± 0.1 | 22 ± 4 | 37 |
| 18b |
|
1.1 ± 0.2 | >300 | >272 |
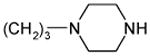
| 20a |
|
1.4 ± 0.2 | 108 ± 11 | 77 |
| 20b |
|
1.7 ± 0.5 | 101 ± 12 | 59 |
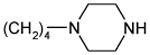
| 22a |
|
1.3 ± 0.5 | 188 ± 17 | 145 |
| 22b |
|
0.8 ± 0.2 | 197 ± 22 | 246 |
| 24a |
|
0.8 ± 0.1 | 9.4 ± 1.0 | 12 |
| 24b |
|
0.6 ± 0.1 | 95 ± 14 | 158 |
IC50 values of compounds to inhibit human MetAPs were shown as mean ± SD of three determinations. The bottom values of all inhibitory dose-response curves were smaller than 10%.
