Abstract
In higher eukaryotes, induced resistance associates with acquisition of a priming state of the cells for a more effective activation of innate immunity; however, the nature of the components for mounting this type of immunological memory is not well known. We identified an extracellular subtilase from Arabidopsis, SBT3.3, the overexpression of which enhances innate immune responses while the loss of function compromises them. SBT3.3 expression initiates a durable autoinduction mechanism that promotes chromatin remodeling and activates a salicylic acid(SA)-dependent mechanism of priming of defense genes for amplified response. Moreover, SBT3.3 expression-sensitized plants for enhanced expression of the OXI1 kinase gene and activation of MAP kinases following pathogen attack, providing additional clues for the regulation of immune priming by SBT3.3. Conversely, in sbt3.3 mutant plants pathogen-mediated induction of SA-related defense gene expression is drastically reduced and activation of MAP kinases inhibited. Moreover, chromatin remodeling of defense-related genes normally associated with activation of an immune priming response appear inhibited in sbt3.3 plants, further indicating the importance of the extracellular SBT3.3 subtilase in the establishment of immune priming. Our results also point to an epigenetic control in the regulation of plant immunity, since SBT3.3 is up-regulated and priming activated when epigenetic control is impeded. SBT3.3 represents a new regulator of primed immunity.
Author Summary
Following a first encounter with a pathogen, higher eukaryotes develop enhanced resistance to subsequent infections by a broad spectrum of pathogens. This type of induced resistance (IR) exhibits memory characteristics after the first encounter with a pathogen —training effect— and appears evolutionarily conserved. IR response components must reside in a plant's capacity to reprogram gene expression. Among the mechanisms involved in immune-related transcriptional reprogramming, the importance of chromatin remodelling is emerging. Recent studies indicated a causal link between priming and chromatin remodelling, pointing to a histone memory for information storage in the plant immune response. These results emphasized the importance of epigenetic control as an additional layer of complexity in plant immunity. However, the nature of the components for activating this type of immunological memory remains elusive. Here, in a search aiming to identify cellular factors integral in regulating immunity in Arabidopsis, we found that the SBT3.3 gene, encoding an extracellular subtilase enzyme, is pivotal for establishing plant immune priming. Moreover, based on molecular and genetic evidences, our results indicate that SBT3.3 expression is under epigenetic control thus highlighting the importance of this mechanism of gene regulation in the control of plant immunity and IR.
Introduction
Plants are continuously faced with threats from pathogenic microorganisms. They counteract microbial infections via activation of an innate immune system in a timely, accurate, and effective manner following pathogen recognition. The innate immune response is thought to act naïvely to individual pathogen encounters and is dependent on the recognition of broadly conserved molecular features, known as microbe-associated molecular patterns (MAMPs), by plasma membrane proteins known as pattern recognition receptors (PRRs). PRR perception of MAMPs at the cell surface leads to a pattern-triggered immune response called PTI [1]. PTI is characterized by the rapid generation of ion fluxes, production of reactive oxygen species (ROS), phosphorylation cascades, and a transcriptional reprograming that favors defense responses over routine cellular requirements [2]. The defense programme is ultimately controlled through the build-up of specific signalling hormone blends, of which salicylic acid (SA) and jasmonic acid (JA) are particularly important, and eventually establish a broad systemic alert state throughout the plant.
Plants develop heightened activation of the innate immune response state resulting from the initial infection manifested in the form of enhanced resistance to subsequent infections by a broad spectrum of pathogens. This type of induced resistance (IR) or cross-protection exhibits memory characteristics after the first encounter with a pathogen - training effect - and appears evolutionarily conserved, even outside the plant kingdom. Netea et al. [3] coined the term “trained immunity” to differentiate it from “innate immunity” (as it is induced only secondarily in hosts that have previously encountered a primary infection), or from “adaptive immunity” (as this implies specificity through T and B cells). In plants, two distinct types of this resistance form have been described: systemic acquired resistance (SAR), and induced systemic resistance (ISR) [4], [5]; both represent a functional immune acclimation requiring the defense response regulator NPR1.
Particularly relevant in IR responses is the observation that defence genes, in both the local (infected) and distal tissue, respond to much lower levels of a pathogenic stimulus in a more rapid and robust manner than controls, thus revealing a “priming” phenomenon. In fact, priming has long been known as a component of IR responses in plants [6], [7] and mammals [8]–[10], and more recently in invertebrates, which like plants lack adaptive immunity [11]. Similarly, Arabidopsis mutants attenuated in pathogen defense (i.e. npr1) are also compromised in priming [12], [13]. Organic and inorganic compounds can also induce this form of resistance in plants [14]. Among these, azelaic acid [15], SA, and its functional analogue benzo(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester (BTH) [16], or the non-protein amino acid β-aminobutyric acid (BABA) [17] have attracted marked interest as they potentiate pathogen-specific defense mechanisms, and induction of a primed state. However, very little is known about the molecular mechanism(s) and signals that set a priming state in motion, or the identity of molecular components that pertain to the maintenance of a long lasting immune primed state, such as SAR.
Conrath et al. [18] hypothesized that IR or cell priming could be built on the accumulation of dormant or inactive signalling proteins, integral in signal amplification that becomes operative following a challenge with another pathogen, thereby initiating signal amplification leading to a faster and stronger activation of defense responses. However, the identity of such signalling components remains elusive. Interestingly, Beckers et al. [12] have shown that during development of BTH induced resistance in Arabidopsis, priming is associated with accumulation of inactive proteins of mitogen-activated protein kinases (MPKs), MPK3 and MPK6. Exposure to the challenges of biotic and abiotic stressors results in stronger activation of the two kinases in primed plants relative to non-primed plants, which is linked to enhanced defense gene expression. Priming of defense gene expression was reduced in mpk3 or mpk6 mutants, showing that pre-stress deposition of a MPK cascade is a critical step in priming plants for a full defense response induction during IR [12].
Essential IR response components must rely in a plant's capacity to reprogram gene expression. Among the mechanisms involved in immune-related transcriptional reprogramming, the importance of chromatin remodeling and covalent histone modifications is emerging [19]. Jaskiewicz et al. [20], reported that during primed BTH immunity, increased acetylation of histone H3 at Lys-9 (H3K9ac) and trimethylation of histone H3 at Lys-4 (H3K4me3) was detected at promoter regions of several SA-responsive genes encoding transcription factors (i.e. WRKY6, WRKY29, and WRKY52). Similarly, constitutively increased H3K4me3 and H3K9ac mark setting in chromatin of the SA-dependent PR1 gene was initially reported in sni1 (suppressor of nrp1-1, inducible 1) mutant [21]. The settling of these histone modifications lead chromatin into a suitable state for efficient SA-responsive gene induction when needed. The results also indicated a causal link between priming and chromatin remodeling, pointing to a histone memory for information storage in the plant stress responses [19]. On the other hand RNA Polymerase V is an enzyme critical in the epigenetic RNA-directed DNA methylation (RdDM) pathway and is involved in regulating both DNA methylation and histone modifications [22]. In this context, López et al. [23] reported that RNA Polymerase V defective mutants carry a constitutive priming phenotype where SA-related defense genes are poised for enhanced activation via similar H3K4me3 and H3K9ac histone modifications in their promoters. These results emphasized the importance of epigenetic control as an additional layer of complexity in plant immunity and IR regulation [23]. Furthermore, DNA methylation has been implicated in the transmission of a priming state or stress memory, endowing progeny of pathogen-inoculated plants with heightened resistance (transgenerational IR), suggesting plants can inherit priming sensitization [24], [25].
In the present study, we report on identification and characterization of the inducible Arabidopsis subtilase SBT3.3 to characterize additional cellular components mediating initiation and or/maintenance of primed immunity. This extracellular proteolytic enzyme serves a signaling role in establishing immune priming. The mechanism subsequently activates chromatin remodeling and defense genes become poised for enhanced activation following pathogen attack. Our study provides strong evidence that SBT3.3 is a primary switch in immune priming, and it may represent one of the missing components in systemic IR establishment.
Results
The Arabidopsis subtilase gene SBT3.3 is up-regulated in the csb3 mutant
The Arabidopsis enhanced disease resistance csb3 (constitutive subtilisin3) mutant [26] was isolated during a search for negative disease resistance regulators in a mutant screening that evaluated constitutive expression of GUS activity driven by the 5′ promoter region of a pathogen-induced subtilase gene (P69C) from tomato plants [27]. Arabidopsis possess fifty-six highly similar genes encoding subtilases [28], therefore constitutive expression of the Arabidopsis gene homologous to P69C would be similarly up-regulated in the csb3 mutant. Constitutively expressed genes differentially expressed in the csb3 mutant with respect to wild-type plants were identified by microarray analysis of RNA transcripts using ATH1 Affymetrix chips. The microarray analysis (NCBI GEO Series number GSE35507) identified one hundred down-regulated genes and 367 up-regulated genes in the csb3 mutant (Supplemental Table S1 and Figure S1). Among the genes up-regulated ≥2-fold (p values<0.05) in the csb3 mutant, we identified 23 genes that could be linked to disease resistance and SA-mediated responses based on published results (Supplemental Table S2). It was notable that among them one encoded a subtilase: SBT3.3 (At1g32960). Moreover, public microarray data mining showed that SBT3.3, out of the 56 paralogous subtilases from Arabidopsis, with the exception of At1g32940, is the one showing strongest response to pathogen attack and to pathogen-related stress signals (Supplementary Figure S2).
Coincident with what has been described in the tomato genome, where the P69C subtilase clusters with three additional P69C-like ORFs (i.e. P69A, P69D, P69C, and P69B) [29], the SBT3.3 subtilase gene was similarly embedded in a genomic cluster encompassing three additional subtilases (i.e. SBT3.5, SBT3.4, SBT3.3 and SBT3.2) in Chromosome 1 (Fig. 1A). Thus, it seems very likely that the Arabidopsis SBT3.3 subtilase, could represent the evolutionarily conserved ortholog of the P69C subtilase from tomato, and the promoter activation was the clue for identifying the csb3 mutant.
Figure 1. SBT3.3 genome organization and induced expression following P.
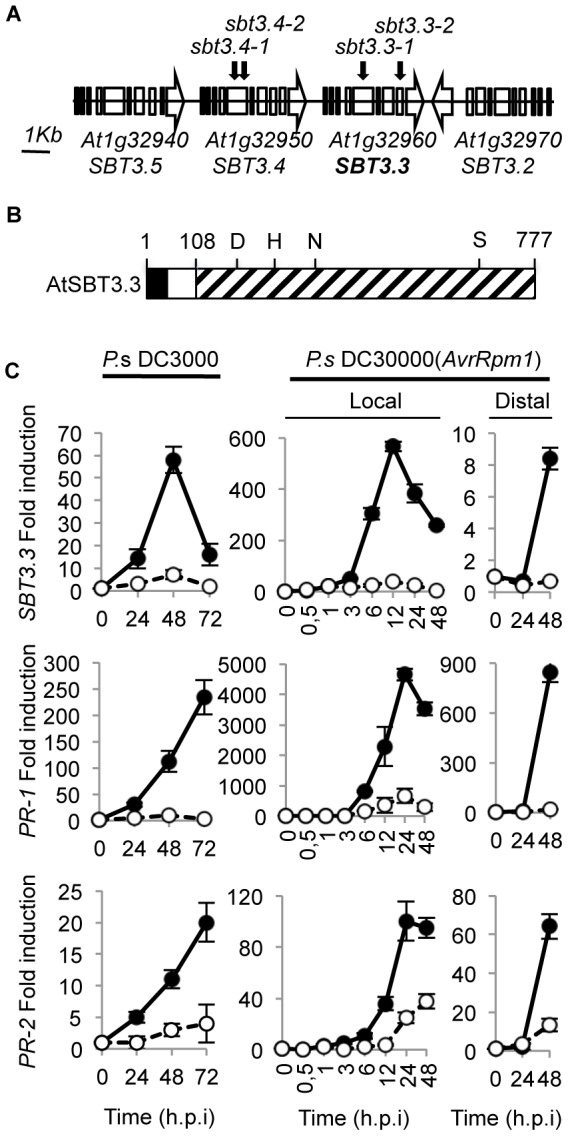
syringae DC3000 infection. A) Four SBT3.3-like open reading frames sequences (named as SBT3.5, SBT3.4, SBT3.3 and SBT3) are arranged in tandem in chromosome I. The distances are only approximate. Arrowheads indicate direction of transcription. Black arrows above the genes show position of T-DNA insertions rendering the sbt3.4 and sbt3.3 mutant alleles. (B) Schematic representation of the SBT3.3 preproenzyme structure. Areas marked respectively in black, white and stippled indicate the signal peptide, propeptide, and mature peptide regions. Numbers depict positions of amino acid residues from the N-terminus. The amino acids forming the catalytic triad (D, H, and S) and the conserved N residues are marked. (C) RT-qPCR analyses showing local induction of SBT3.3, PR-1, and PR-2 gene expression upon infection with virulent PsDC3000, and both local and distal induction following infection with the avirulent PsDC3000 (AvrRpm1) strain. Filled circles represent inoculated plants, and empty circles represent mock-inoculated plants (controls). Data represent mean ± SD, n = 3 replicates. Expression was normalized to the constitutive ACT2 gene, then to expression at time 0 in Col-0 plants.
The SBT3.3 gene encodes a 777 amino acid preproenzyme (Fig. 1B and Supplemental Figure S3) containing a N-terminal 25 amino acid signal peptide followed by an 86-amino acid propolypeptide (aa 26 to 111), and a 666-amino acid mature polypeptide with a predicted molecular weight of 71237 Da. The mature polypeptide comprises eight potential asparagine-linked glycosylation sites (NXS/T). On the basis of sequence similarities with other subtilases, including P69C [30], the amino acid residues Ser-555, Asp-145, and His-223 were identified as residues of the catalytic triad (Fig. 1B).
Expression pattern of SBT3.3 following pathogen inoculation
To mode of SBT3.3 gene regulation in plant immunity was assessed by inoculating Col-0 leaves with the bacterial pathogen Pseudomonas syringae DC3000 (PsDC3000), carrying or not the avirulence gene AvrRpm1, and temporal gene expression patterns were determined by quantitative RT-PCR (RT-qPCR). SBT3.3 was barely detectable in mock-inoculated plants, but strongly induced during the PsDC3000 immune response (Fig. 1C). However, inconsistent with observations for SA-regulated marker genes (i.e. PR-1 and PR-2), SBT3.3 induction was transient, peaking at 48 hpi (hours post inoculation) and abruptly decaying thereafter. The strongest induction was observed following inoculation with the avirulent strain PsDC3000 (AvrRpm1) (Figure 1C); induction was again transient, peaking at 12 h.p.i, and decayed thereafter. SBT3.3 expression preceded PR-1 and PR-2 gene induction, suggesting that the signals that set in motion transcriptional reprogramming of these two types of gene responses might differ. Expression of PR-1 and PR-2 genes in the distal non-inoculated leaves was also observed for SBT3.3, although distal expression was not as high as that attained in local leaves (Fig. 1C). High and rapid (within an hour) induced SBT3.3 expression was also promoted by bacterial PAMP flg22 application to Col-0 plants (Supplemental Fig. S4), providing additional support for the association of SBT3.3 expression with early innate immune response activation.
SBT3.3 functions in disease resistance
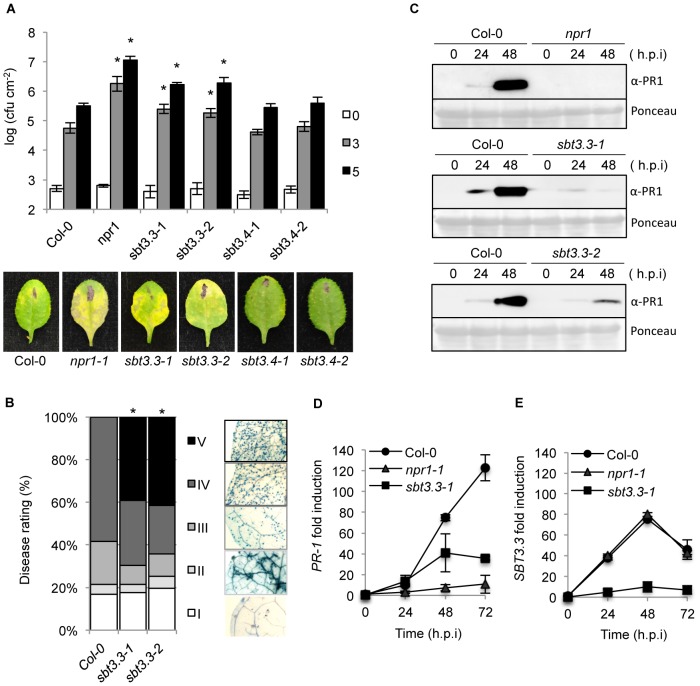
The importance of SBT3.3 in plant immunity was investigated by characterizing the response of two independent T-DNA insertion lines for SBT3.3 (sbt3.3-1 and sbt3.3-2; Fig. 1A) to PsDC3000 infection (Fig. 2A). We also characterized the response of two independent T-DNA lines available for one of the linked subtilase genes (i.e. SBT3.4) within the same genomic cluster (sbt3.4-1 and sbt3.4-2; Fig. 1A). The enhanced disease susceptibility mutant npr1-1 was incorporated into the experiments as a control. Disease performance was assayed by measuring bacterial growth in the inoculated leaves (Fig. 2A). The two control mutant lines, sbt3.4-1 and sbt3.4-2 behaved as inoculated Col-0 plants, indicating that SBT3.4 is not essential to activate immune responses. However, npr1 plants, and either one of the two sbt3.3 mutants, supported significant increases in bacterial growth. The enhanced disease susceptibility was accompanied by development of disease symptoms in the form of visible chlorotic lesions on inoculated leaves (Fig. 2A). The results suggest that SBT3.3 positively regulates disease resistance to PsDC3000.
Figure 2. SBT3.3 loss of function increases disease susceptibility to P. syringae DC3000 and H. arabidopsidis.
(A) Five-week-old plants were inoculated with PsDC3000. Zero (white bars), three (grey bars) and five (black bars) days after inoculation, the bacterial growth was measured. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test. Below are representatives of inoculated leaves of the indicated genotypes. (B) Quantification of H. arabidopsidis conidia development on Col-0, and sbt3.3-1 and sbt3.3-2 mutants. Asterisks indicate statistically different distributions of disease severity classes compared with Col-0 plants (χ2 test; α = 0.05). (C) Western blots with anti-PR1 antibodies reveals inhibition of PR1 induced accumulation in nrp1, sbt3.3-1 and sbt3.3-2 mutant plants, compared to Col-0, following inoculation with PsDC3000. The experiments were repeated three times with similar results. (D–E) Time-course RT-qPCR analysis showing PR-1 (D) and SBT3.3 (E) gene expression in Col-0, sbt3.3-1, and npr1-1 plants after infection with PsDC3000. Data represent the mean ± SD; n = 3 replicates and gene expression given as in Fig. 1.
Changes in the susceptibility of sbt3.3 plants to biotrophic pathogens were further investigated by inoculating plants with a virulent strain of the obligate oomycete Hyaloperonospora arabidopsidis (isolate Noco) (Fig. 2B). Disease severity was assessed at 7 d.p.i in lactophenol trypan-blue-stained leaves. The leaves were classified into five categories (I to V) according to their degree of colonization by the oomycete (Fig. 2B). Both, sbt3.3-1 and sbt3.3-2 mutant plants exhibited a significantly higher degree of colonization by the oomycete than the control Col-0 plants (Fig. 2B), becoming heavily covered with sporangiophores, which elicited appearance of chlorosis and eventual leaf collapse (namely Class V). The observed enhanced disease susceptibility of sbt3.3-1 and sbt3.3-2 plants to H. arabidopsidis was corroborated by directly counting of spore production in inoculated plants (Supplemental Fig. S5). These results confirmed that loss of SBT3.3 function also enhanced plant susceptibility to H. arabidopsisdis, further substantiating its value in establishing an effective plant immune response.
SBT3.3 is required for expression of SA-responsive genes
Compromised expression of SA-responsive genes is observed in mutants defective in resistance to biotrophic pathogens (i.e. npr1; [31]). Consequently, we considered the possibility that the increased susceptibility towards pathogens observed in SBT3.3 defective mutants might be similarly accompanied by a compromised expression of SA-responsive genes. Therefore, induction of PR-1 accumulation was examined by Western blot in sbt3.3, npr1, and Col-0 plants following inoculation with PsDC3000. The PR-1 protein, as expected, was nearly absent in npr1 plants, even at 48 hpi with PsDC3000 (Fig. 2C), while PR-1 accumulation was notable in Col-0 following inoculation. Interestingly, sbt3.3-1 and sbt3.3-2 plants exhibited results similar to the npr1 mutant, showing a notable impediment to induced PR-1 protein accumulation post pathogen inoculation (Fig. 2C). These results were confirmed at the transcriptional level by measuring PR-1 transcript level by RT-qPCR (Fig. 2D). As for NPR1 being required for full immunity, our results suggest that SBT3.3 is required for full expression of downstream SA-responsive genes. This helps explaining why mutants defective in SBT3.3 are compromised in disease resistance (Figure 2A and B).
SBT3.3 expression is SA-independent and responds to H2O2
The same mRNA preparations shown in Figure 2D were used to quantify SBT3.3 transcript accumulation following inoculation with PsDC3000 in Col-0 and npr1 plants (Fig. 2E). RNA preparations from sbt3.3 plants (here used as a control) served to demonstrate that in the mutant induced SBT3.3 expression was drastically down-regulated due to the T-DNA insertion. In marked contrast with the substantial reduction observed for PR-1 activation (Figure 2D), SBT3.3 expression in npr1 plants was identical to that attained in Col-0 plants. Furthermore, in sid2-1 mutant plants (defective in SA synthesis) induction of SBT3.3 expression upon inoculation with PsDC3000(AvrRpm1), remained unchanged with respect to Col-0 plants (Fig. 3A). This differs with the compromised expression of PR-1 occurring in sid2 plants (Fig. 3A). These contrasting differences indicated that for pathogen-induced SBT3.3 expression, SA synthesis and its perception through NPR1 are dispensable.
Figure 3. SBT3.3 gene expression is SA-independent and rapidly induced by H2O2.
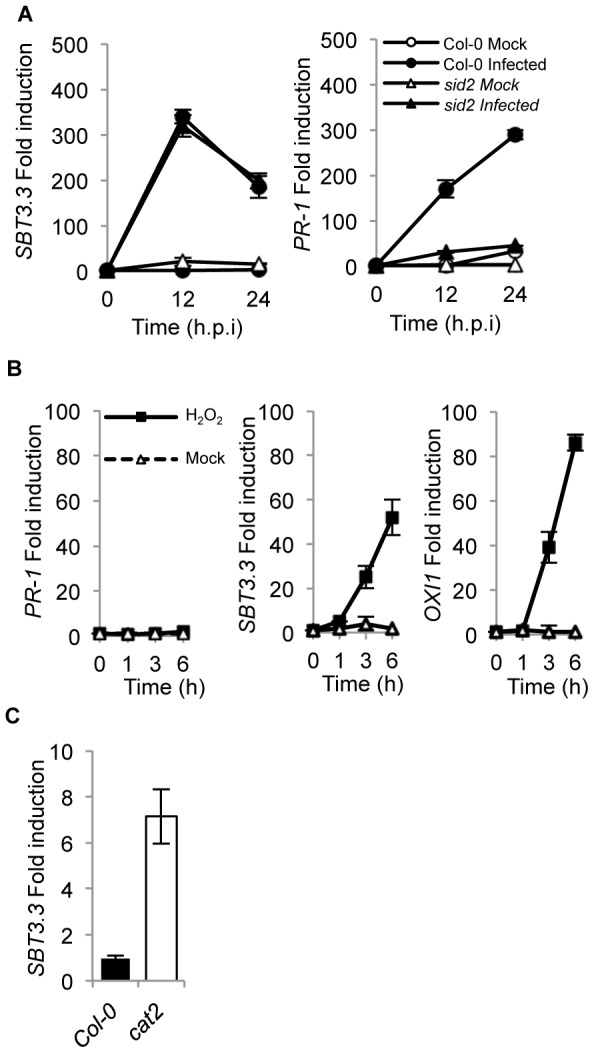
(A) RT-qPCR analysis showing SBT3.3 gene expression in mock- (white symbols) and PsDC3000 (AvrRpm1)-inoculated (black symbols) leaves in Col-0 (circles) and sid2-1 (triangles). (B) RT-qPCR analysis showing PR-1, SBT3.3 and OXI1 gene expression in mock- (white triangles), and H2O2-treated (black squares) Col-0 seedlings. (C) SBT3.3 expression level in a cat2 mutant. Data represent the mean ± SD; n = 3 replicates and gene expression given as in Fig. 1.
Since oxidative burst and concurrent H2O2 accumulation preceded SA build-up during basal immunity activation [32] and SBT3.3 induction appeared as an early event preceding PR gene induction by SA (Fig. 1C–D), we hypothesized that H2O2 could mediate SBT3.3 induction. In fact, spraying Arabidopsis leaves with a 1 mM solution of H2O2 elicited a rapid SBT3.3 induction which was notable at 1 to 3 hours after treatment (Fig. 3B). Similarly, expression of the OXI1 gene, which encodes a kinase highly induced under oxidative stress conditions [33] was triggered by H2O2 (Fig. 3B). However, under similar inductive conditions expression of the SA-regulated gene PR-1 remained unchanged (Fig. 3B). Moreover, the cat2 mutant defective in the dismutation of H2O2 and exhibiting enhanced H2O2 accumulation, revealed increased SBT3.3 expression compared to Col-0 (Fig. 3C). These observations indicated that SBT3.3 activation might result from early H2O2 production during the immune response.
SBT3.3 is secreted and accumulates extracellularly
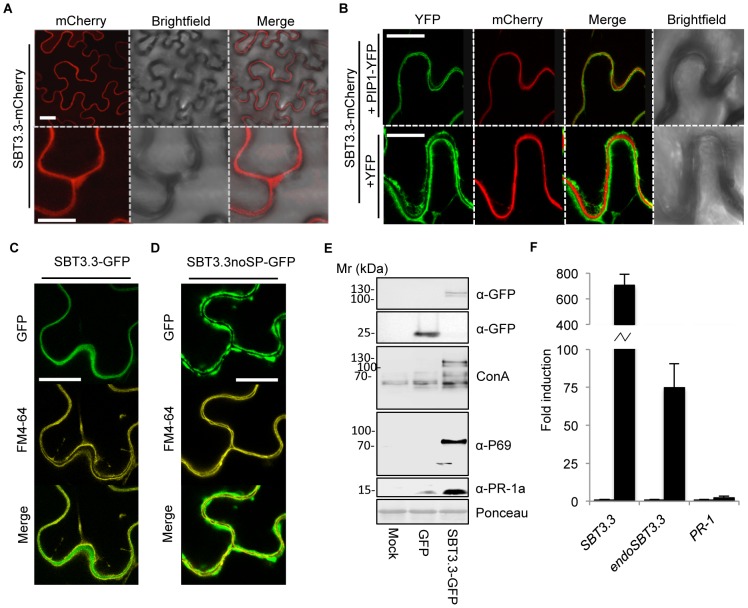
Plant subtilases are synthesized in the form of preproprotein precursors, translocated via a signal peptide into the endomembrane system, and activated through further cleavage of the propeptide [34]. Most plant subtilases are considered glycoproteins that predominantly accumulate extracellularly [30], [34], [35]. SBT3.3 subcellular localization was determined by fusing monomeric cherry fluorescent protein (mCherry) to the C-terminus of the full length SBT3.3, and the construct, driven by 35S promoter, expressed in Nicotiana benthaminana leaves using agro-infiltration. Localization of the fusion protein was confirmed by confocal microscopy. Results showed that SBT3.3-mCherry fluorescence was uniformly distributed in the pericellular apoplastic space (Fig. 4A). Similar pericellular localization was observed in transgenic Arabidopsis plants expressing a 35S::SBT3.3-GFP gene construct (Supplemental Fig. S6). The mCherry-tagged subtilase was co-expressed with either a construct bearing the plasma membrane integral protein PIP1 fused to YFP, or alternatively with a free cytosolic YFP protein to more precisely define its localization. SBT3.3-mCherry was localized externally to the cytoplasm, as revealed when co-expressed with a free cytosolic YFP (Fig. 4B). Furthermore, SBT3.3-mCherry was found to be sandwiched between the PIP1-YFP-tagged plasma membrane marker of adjacent cells (Fig. 4B) and thus unambiguously localized to the extracellular matrix. SBT3.3 extracellular localization was also confirmed upon expression of a SBT3.3-GFP protein fusion in the presence of FM4-64, a plasma membrane specific fluorescent dye (Fig. 4C). Furthermore, expression of an SBT3.3noSP-GFP gene construct in which the N-terminal 25 amino acid signal peptide of SBT3.3 has been deleted, revealed that the SBT3.3noSP-GFP protein fusion was not secreted to the extracellular matrix and was retained in the cytoplasm, as delineated by the co-localization with FM4-64 (Fig. 4D). These results indicate that SBT3.3 is secreted and accumulated in the plant extracellular matrix, and secretion depends on the presence of its signal peptide.
Figure 4. Extracellular localization of SBT3.3-mCherry in N. benthamiana leaves by confocal microscopy.
(A) Expression of SBT3.3-mCherry (50 hpi) results in a uniform extracellular fluorescence. (B) Co-expression of SBT3.3-mCherry with the plasma membrane marker PIP-YFP (upper panel) and with free cytosolic YFP (lower panel). (C) Co-localization of SBT3.3-GFP with the plasma membrane fluorescent marker FM4-64. (D) Co-localization of SBT3.3noSP-GFP with the plasma membrane fluorescent marker FM4-64. Scale bars are 10 µm (A-upper panel), 40 µn (A-lower panel, B, C and D panels). (E) Western blots of total protein extracts from N. benthamiana leaves transitorily overexpressing GFP alone or a SBT3.3-GFP fusion proteins revealed with either concanavalin A, or with anti-GFP, anti-P69 and anti-PR-1a antibodies. Total protein extracts from empty A. tumefaciens agroinfiltrated N. benthamiana leaves (mock) were used as controls. (F) Overexpression of SBT3.3 under the control of a 35S promoter in stable transgenic Arabidopsis plants triggers activation of the endogenous SBT3.3 gene. Accumulation levels of the 35S driven SBT3.3 transcripts (SBT3.3), the endogenous SBT3.3 transcripts (endoSBT3.3) and PR-1 transcripts were measured comparatively in healthy Col-0 plants (left bars) and in a transgenic 35S::SBT3.3OEX line (right black bars). Data represent the mean ± SD; n = 3 biological replicates and gene expression given as in Fig. 1.
SBT3.3 expression in N. benthamiana activates expression of an endogenous P69 subtilase
Chichkova et al. [36] demonstrated that following agro-infiltration with a GFP-tagged phytaspase, an Arabidopsis cell death-associated subtilase of similar size to SBT3.3; two proteins of ∼110 and ∼120 kD corresponding to the mature and the pro-protein phytaspases, respectively, accumulated in crude extracts [34]. Following expression of SBT3.3-GFP, two similar protein bands of ∼110 and ∼120 kD were detected in Western blots using an anti-GFP antibody (upper panel; Fig. 4E). The difference between the theoretical mature 98,2 kD SBT3.3-GFP fusion and the observed mature 110 kD proteins must be due to posttranslational modifications. In fact, glycosylation was early proposed to regulate activity of plant subtilases [37], [38]. We performed Western blots of the same leaf extracts and developed the nitrocellulose filters with Concanavalin A (Con A) coupled to horseradish peroxidase to identify if SBT3.3 is glycosylated. These analyses revealed that Con A recognized the 120/110 kD doublet (Fig. 4E) in extracts expressing the SBT3.3-GFP construct, thus confirming that SBT3.3 is glycosylated. Interestingly, Con A also recognized a band of ∼70 kD that only accumulated following SBT3.3-GFP expression (Figure 4E). This ∼70 kD band was reminiscent of a glycosylated 69 kD P69 subtilase conserved in the Solanaceous species [27], [30]. To verify this possibility, Western blots were developed with anti-P69C antibodies [30]. Results revealed that the ∼70 kD Con-A reacting band was recognized by anti-P69 antibodies (Fig. 4E) indicating that SBT3.3-GFP signaled tobacco cells to activate expression of an endogenous P69 subtilase homologue. Interestingly, when we extended this analysis to other defense-related proteins, i.e. by using antibodies against the PR-1a isoform from tobacco, we observed that overexpression of SBT3.3-GFP similarly triggered SA-responsive PR-1a protein accumulation (lower panel; Fig. 4E). We subsequently created a missense mutant in the SBT3.3-GFP “catalytic triad” (S555A; SBT3.3m-GFP), to ascertain if the observed signaling required integrity of the subtilase proteolytic activity. Expression of the missense mutant in N. benthamiana no longer promoted local accumulation of the corresponding endogenous P69 subtilase, or the PR-1a protein (Supplemental Figure S7). All of these observations are consistent with a model in which Arabidopsis SBT3.3 subtilase local expression autonomously triggers immune-like responses in a heterologous system, and the serine proteolytic activity of the subtilase is necessary for this effect. As occurs in SBT3.3OEX1 plants (see below), expression of the SBT3.3-GFP gene construct in transgenic Arabidopsis plants conferred enhanced disease resistance to PsDC3000 and is in contrast with the lack of effect observed for the SBT3.3m-GFP construct (Supplemental Figure S8). Moreover, the same SBT3.3-GFP gene construct is able to abrogate the characteristic enhanced disease susceptibility phenotype of sbt3.3 plants, conferring enhanced disease resistance to PsDC3000 to the stably transformed sbt3.3 mutant plants (Supplemental Figure S9). This further indicates functionality of SBT3.3-GFP fusion protein in promoting immune responses in Arabidopsis.
SBT3.3 artificial expression in transgenic Arabidopsis triggers expression of the endogenous gene
The above observations indicated that SBT3.3 promotes the expression and accumulation of a homologous subtilase (i.e. P69) in N. benthamiana. We studied a stable transgenic Arabidopsis line constitutively expressing SBT3.3 under the control of the 35S promoter (SBT3.3OEX) by measuring activation on the corresponding endogenous SBT3.3 gene to test if the same phenomenon could be reproduced in Arabidopsis. Antibodies against SBT3.3 were not available; therefore we instead performed RT-qPCR measurements using 2 different pairs of oligonucleotides. One of those pairs discriminates between the endogenous SBT3.3 mRNAs (endoSBT3.3), transcribed from its own gene, and the other pair was designed to measure the whole amount of SBT3.3 mRNAs (SBT3.3). In Col-0 plants, as expected, both SBT3.3 and endoSBT3.3 transcript expression was very low (Fig. 4F). In contrast, in the transgenic SBT3.3OEX line, SBT3.3 transcript accumulation was prominent (Fig. 4F), and importantly, this was also followed by a high endoSBT3.3 transcript accumulation. This effect gives support to the hypothesis that SBT3.3 expression is able per se to signal its own gene activation. This induction appears “self” controlled, since endogenous PR-1 transcript levels in transgenic plants do not exhibit significant variation with respect to Col-0 plants (Fig. 4F). However, in agro-infiltrated tobacco leaves we observed that SBT3.3-GFP expression promotes accumulation of both the endogenous P69 subtilase homolog, and the SA-dependent PR-1a protein. This difference can only be explained by noting that in the experiments with N. benthamiana, A. tumefaciens is inevitably present, which in turn may supply PAMPs in collaboration with SBT3.3, triggering a downstream SA signaling pathway, and in turn SA-defense related gene activation. If this mechanism operated effectively, then SBT3.3 would be required to facilitate early immune signaling preceding defense response activation.
SBT3.3 overexpression confers enhanced disease resistance to PsDC3000 and H. arabidopsidis
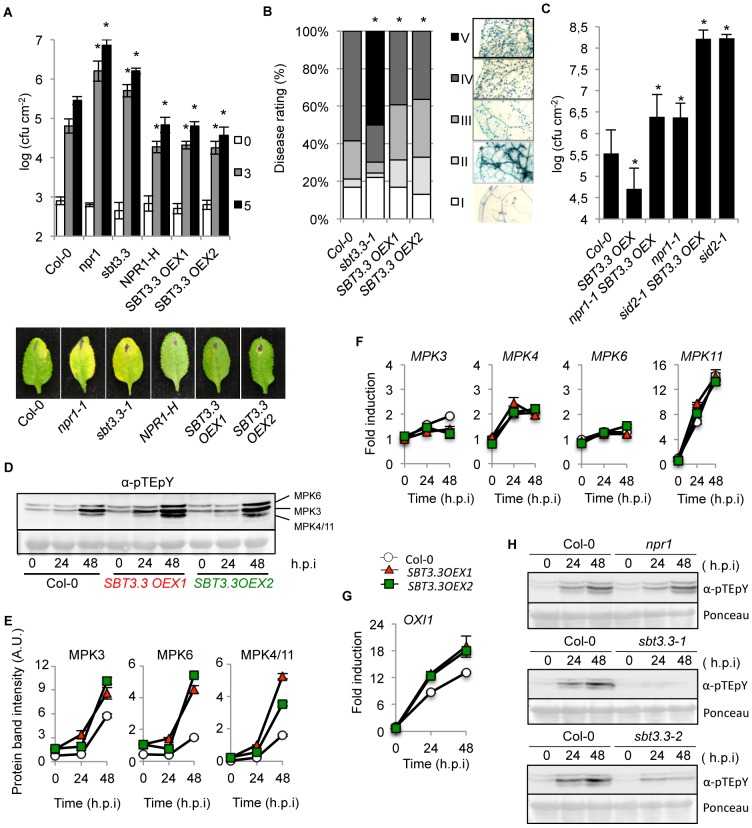
Two independent Arabidopsis transgenic lines that overexpress SBT3.3 were inoculated with PsDC3000 and disease response recorded to further assess the role of SBT3.3 subtilase in plant immunity (i.e. SBT3.3OEX1 and SBT3.3OEX2). The enhanced disease resistance line overexpressing the NPR1 regulator (NPR1-H, [39]), one of the SBT3.3 defective mutants (i.e. sbt3.3-1), and Col-0 were included in this experiment for comparison. Figure 5A shows that the two SBT3.3OEX lines exhibited significant enhanced disease resistance responses to PsDC3000 compared to Col-0. This enhanced resistance was of a magnitude similar to that attained by NPR1-H plants. In contrast, the sbt3.3-1 mutant reproduced the expected increased disease susceptibility previously shown in Figure 2.
Figure 5. SBT3.3 overexpression confers enhanced disease resistance and enhanced mitogen-activated kinase activation.
(A) Plants of the indicated genotype were inoculated with PsDC3000. Zero (white bars), three (grey bars) and five (black bars) days after inoculation, the bacterial growth was measured. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) analysed with a Student's t-test. Below are shown representative pictures of leaves of the inoculated plants genotypes. (B) Quantified H. arabidopsidis conidia development on leaves of the indicated genotypes. Asterisks indicate statistically different distributions of disease severity classes compared with Col-0 plants (χ2 test; α = 0.05). (C) Disease resistance phenotype of homozygous double npr1 SBT3.3OEX1 and sid2 SBT3.3OEX1 mutant plants against PsDC3000 was compared to Col-0 and to their respective parental lines. Experiments were performed as described in Figure 2A. (D) Western blot with anti-pTEpY antibodies of crude protein extracts derived from Col-0, SBT3.3OEX1 and SBT3.3OEX2 plants at 0, 24, and 48 h.p.i with PsDC3000. Equal protein loading was check by Ponceau staining of the nitrocellulose filter. MAP6, MPK3 and MPK4/11 migrating bands are indicated on the right. The experiments were repeated three times with similar results. (E) Densitometric scan quantification of protein bands corresponding to MPK3, MPK6 and MPK4/11 bands as shown in (D) following inoculation of Col-0 (white), SBT3.3OEX1 (red) and SBT3.3OEX2 (green) plants with PsDC3000. Data represent the mean ± SD; n = 3 replicates. (F–G) Time-course RT-qPCR analysis showing MPK3, MPK4, MPK6, MPK11 (F) and OXI1 (G) gene expression in the indicated genotypes following inoculation with PsDC3000. Data represent the mean ± SD; n = 3 replicates. Expression was normalized to the constitutive ACT2 gene expression as in Fig. 1. (H). Western blot with anti-pTEpY antibodies of crude protein extracts derived from Col-0, npr1, sbt3.3-1 and sbt3.3-2 plants at 0, 24, and 48 h.p.i with PsDC3000. Equal protein loading was check by Ponceau staining of the nitrocellulose filter. The experiments were repeated three times with similar results.
The SBT3.3 overexpressing lines exhibited an enhanced disease resistance phenotype when exposed to H. arabidopsidis (Fig. 5B). The two lines overexpressing SBT3.3 exhibited a significantly lower colonization of the oomycete than the control Col-0 plants or the highly susceptible sbt3.3-1 mutant (Fig. 5B). The observed enhanced disease resistance of sbt3.3-1 to H. arabidopsidis was corroborated by directly counting of spore production in inoculated plants (Supplemental Fig. S5). The observed heightened resistance against these two pathogens indicated that SBT3.3 functions as a positive plant immunity regulator. Furthermore, when the SBT3.3 overexpression phenotype was assayed in an nrp1 or sid2 mutant background, the enhanced disease resistance to PsDC3000 was abrogated (Fig. 5C). These results indicate that SBT3.3, as a positive plant immunity regulator, operates upstream of the SA pathway.
SBT3.3 expression confers enhanced activation of MPK kinases
Elevated mitogen-activated protein kinases (MAPKs) activation is genuinely linked to IR development [12], and in general to innate immune responses [40], [41], [42]. Therefore, our next objective was to demonstrate if the enhanced resistance phenotype mediated by the sole SBT3.3 subtilase expression could elicit elevated MPKs activation. We subsequently employed an antibody recognizing the phosphorylated residues within the MAPK activation loop (the so called pTEpY motif, where p denotes the phosphorylated residue). Western blot analysis of protein extracts derived from healthy Col-0 plants or from two SBT3.3OEX lines revealed positive immunoreactive signals in two polypeptides corresponding to MPK6 and MPK3 [42]. Following densitometric scanning of Western blots, the two immunoreactive bands appeared moderately more intense in the overexpression lines relative to Col-0 control lines (Fig. 5D–E). Inoculation with PsDC3000 promoted a further activation-associated dual TEY phosphorylation of MPK3 and MPK6, which was higher in the two SBT3.3OEX lines than in Col-0 plants (Fig. 5D–E). In addition, MPK4/MPK11, which migrated as a single band on SDS-PAGE [42], became activated only following bacterial inoculation, and activation was again more intense in the two SBT3.3OEX lines. Therefore, dual phosphorylation of the TEY amino acid motif within the MPK activation loop, which is required for kinase activity appeared increased in plants expressing SBT3.3. However, despite these differences at the protein level, no significant differences were detected with respect to transcript accumulation induction for these MPKs between Col-0 and SBT3.3OEX lines (Fig. 5F).
OXI1 is a serine/threonine kinase of the AGC protein kinase family required for oxidative burst-mediated signaling in Arabidopsis; its expression was consistent with that of SBT3.3, and was induced by H2O2 ([33]; and Fig. 3B). OXI1 was required for MPK3 and MPK6 activation and for basal resistance to H. arabidopsidis [33]. In view of these observations, we hypothesized that the imposed SBT3.3 expression might sensitize cells to bring earlier or higher OXI1 expression levels following pathogen infection, and in turn provide an explanation for the higher activation observed in MPKs. We measured OXI1 comparative transcript level between Col-0 and two SBT3.3OEX lines following PsDC3000 infection by RT-qPCR. Results indicated the two SBT3.3OEX lines expressed OXI1 to higher levels than Col-0 (Fig. 5G). This offers a viable explanation as to why MPKs exhibited increased activation in SBT3.3OEX plants following pathogen attack, even in the absence of differential gene expression relative to Col-0. Moreover, in sbt3.3 mutant lines MPKs activation following inoculation with PsDC3000 was drastically reduced in comparison to Col-0 (Figure 5H). This observation reinforces the consideration that SBT3.3 appears to function as a positive regulator of the pathway leading to activation of MAP kinases. Interestingly, MPKs activation was not altered in the enhanced disease susceptibility nrp1 mutant (Figure 5H) and neither was the expression of the SBT3.3 gene altered in this same mutant (Figure 3F). This served as a control towards the specific requirement of SBT3.3 for MAKs activation and suggest that this specific signal module operates upstream of the NPR1 regulator.
SA-mediated defense genes are poised for enhanced activation in plants overexpressing SBT3.3
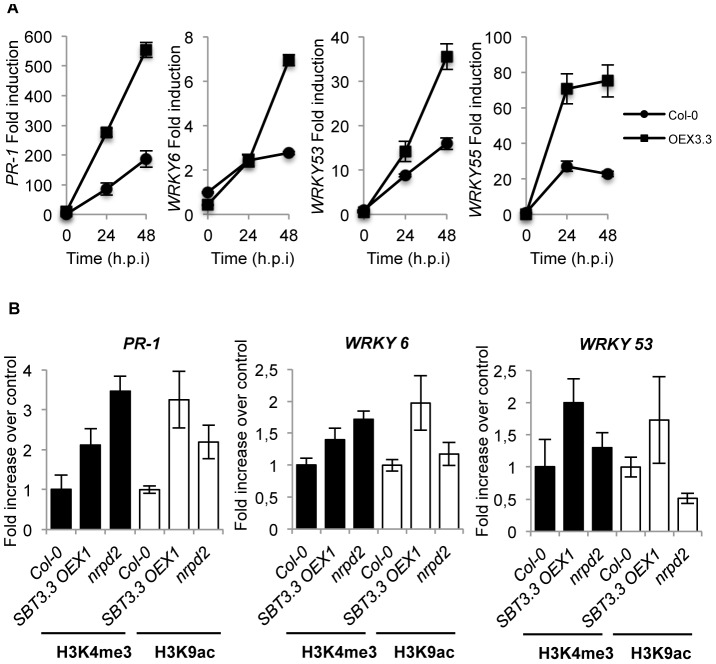
The above results prompted us to search if SA-dependent genes were poised for increase activation following SBT3.3 expression. Therefore, we inoculated Col-0 plants, and one SBT3.3OEX line with PsDC3000, and compared PR-1 expression patterns. Interestingly, after pathogen inoculation induction of PR-1 gene expression showed a notorious enhancement in SBT3.3OEX plants when compared to Col-0 (Fig. 6A). Moreover, the genes encoding the transcription factors WRKY6, WRKY53, and WRKY35, mediating transcriptional regulation of SA-related genes, including PR-1 [43], themselves induced by pathogen infection [44], showed similar enhanced induced expression in SBT3.3OEX plants (Fig. 6A). Thus, SBT3.3 mediated poising of defense genes for enhanced activation following perception of a pathogenic cue, invoking a role for SBT3.3 in priming immune responses.
Figure 6. SBT3.3 expression poises SA-mediated defense genes for enhanced activation.
(A) RT-qPCR of PR-1, WRKY6, WRKY53 and WRKY55 transcript levels following inoculation with PsDC3000 in Col-0 and SBT3.3OEX1 plants. Data represent the mean ± SD; n = 3 replicates. (B) Comparative level of histone H3 Lys4 trimethylation (H3K4me3) and histone H3 K9 acetylation (H3K9ac) on the PR-1, WRKY6 and WRKY53 gene promoters as present in leaf samples from Col-0, SBT3.3OEX1 and nrpd2 plants. Data are standardized for Col-0 histone modification levels. Data represent the mean ± SD; n = 3 biological replicates. Expression was normalized to the expression of the constitutive ACT2 gene.
Poising SA–related genes and primed immunity concur in plants defective in the RdDM pathway, such as mutants affected in different subunits of the RNA Pol V (i.e. nrpd2) [23], and also following pharmacological treatment with the priming agent BTH [20]. In both cases, chromatin histone activation marks appeared enriched in SA-related gene promoters. Consequently, we hypothesized that following expression of SBT3.3 SA-related defense genes could be poised for enhanced activation by differential histone modification. By using chromatin immunoprecipitation (ChIP), we analyzed H3 Lys4 trimethylation (H3K4me3) and H3 Lys9 acetylation (H3K9ac) on the PR-1, WRKY6 and WRKY53 gene promoter region in both Col-0 plants and SBT3.3OEX plants. The enhanced disease resistant mutant nrpd2, defective in RNA PolV activity and compromised in the RdDM pathway [23], was included as a control. On the PR-1 promoter, H3K4me3 and H3K9ac activation marks increased more than two and three-fold, respectively in SBT3.3 overexpressing plants when compared to Col-0 (Fig. 6B). The two activation marks were similarly increased in nrpd2 plants, with only some differences in intensity (Fig. 6B). As for PR-1, the histone marks also showed increases in the WRKY6 and WRKY53 promoters in SBT3.3 overexpressing plants, and to a lesser extent in nrpd2 plants, relative to Col-0 plants (Fig. 6B). Therefore, chromatin marks normally associated with active genes abound in the promoter regions of SA-related genes in SBT3.3 overexpressing plants, although gene activation does not occur in these plants. The marks appear to serve as an on-switch for priming, and helps explain why the same genes show enhanced induction in SBT3.3OEX plants upon pathogenic attack (Fig. 6A).
SBT3.3 expression increases H3K4me3 activation marks in its own promoter
Results showed the sole expression of SBT3.3 in transgenic Arabidopsis plants was able to promote activation of the endogenous gene (Figure 4F). Therefore, SBT3.3 per se might be signaling chromatin remodeling of its own promoter as it does for the PR-1 gene promoter (Fig. 6B). ChIP for H3K4me3 and H3K9ac marks at the SBT3.3 promoter region in SBT3.3 overexpressing plants revealed that the H3K4me3 mark was notably increased compared to Col-0 plants, and moreover, the enhancement in H3K4me3 marks was mirrored in nrpd2 plants (Fig. 7A). However, H3K9ac marks in the SBT3.3 gene promoter did not increase in the SBT3.3 overexpressing plants and the nrpd2 mutant when compared to Col-0 (Fig. 7A). These results contrasted with the common increase of both activation marks in the PR-1 gene (Fig. 6B), and suggested the existence of specific histone codes regulating gene expression. Alternatively, because the increase in H3K4me3 activation marks observed in the SBT3.3 gene promoter between nrpd2 and SBT3.3OEX plants were matched, we reasoned that nrpd2 plants might also carry constitutive SBT3.3 gene expression. SBT3.3 transcript level determination by RT-qPCR in Col-0 and nrpd2 plants showed the mutant constitutively expressing SBT3.3 (Fig. 7B). These results suggest that SBT3.3 expression is under negative epigenetic control, and expression is relieved following inhibition of RdDM. In fact, treatment of Col-0 seedlings with sulfamethazine (SMZ), a chemical suppressor of epigenetic gene silencing (i.e. RdDM) that derepress silenced genes [45], relieved SBT3.3 and promoted transcript accumulation (Fig. 7C), to levels similar to those attained in nrpd2 plants. These observations therefore support the contention of an epigenetic control towards SBT3.3, and indicate that SBT3.3 acts as a positive regulator of a priming phenomenon for more efficient deployment of immune responses. In addition, a low concentration (100 µM) pharmacological treatment with of the priming agent BTH administered to Col-0 plants promoted the enhanced deposition of H3K4me3 and H3K9ac marks in the PR-1 gene promoter, and to a minor extent also in WRKY6 and WRKY53 gene promoters (Fig. 7D), as was observed in previous studies [20], [23]. Similarly, BTH also induced H3K4me3 activation marks, and to a less extent also of H3K9ac marks, in the SBT3.3 gene promoter in Col-0 plants. Thus, as a priming agent, BTH not only induced chromatin remodeling of SA-related genes similar to the RdDM-defective and enhanced resistance mutant nrpd2; it also mimicked SBT3.3 chromatin remodeling triggered by the SBT3.3 itself.
Figure 7. SBT3.3 expression promotes chromatin remodeling and is under epigenetic control.
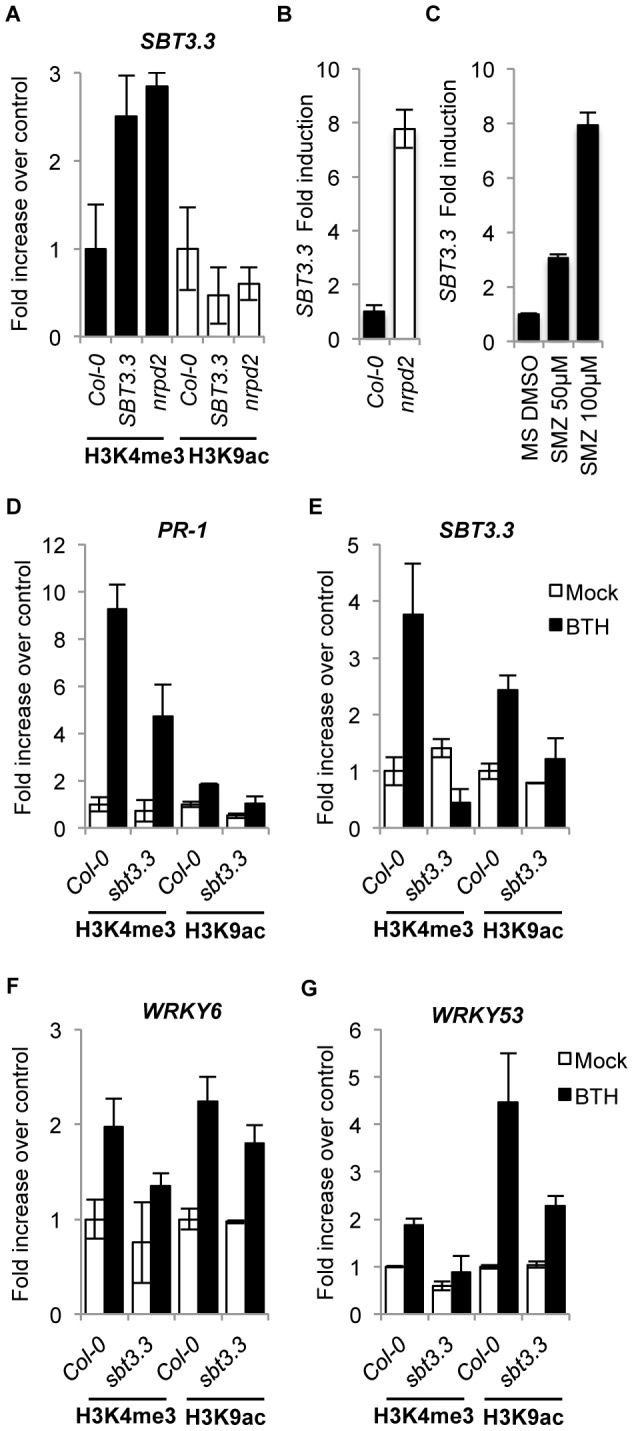
(A) Comparative level of H3K4me3 and H3K9ac mark setting on the SBT3.3 gene promoter as present in leaf samples from Col-0, SBT3.3OEX1 and nrpd2 plants. Data are standardized for Col-0 histone modification levels. Data represent the mean ± SD; n = 3 biological replicates. (B) RT-qPCR of SBT3.3 transcript levels in Col-0 and nrpd2 plants. Data represent the mean ± SD; n = 3 biological replicates. Expression was normalized to the expression of the constitutive ACT2 gene and then to the expression in Col-0 plants. (C) RT-qPCR of SBT3.3 transcript levels in Col-0 seedlings upon treatment with 50 µM and 100 µM of sulfamethazine (SMZ) compared to mock (DMSO). Data represent the mean ± SD; n = 3 biological replicates. Expression was normalized to the expression of the constitutive ACT2 gene, then to mocked Col-0 plant expression. (D–G) Comparative level of H3K4me3 and H3K9ac mark setting on PR-1 (D), SBT3.3 (E), WRKY6 (F) and WRKY53 (G) gene promoters in Col0 and sbt3.3 plants following treatment with 100 µM BTH compared to buffer alone (mock). Data represent the mean ± SD; n = 3 biological replicates.
The importance of SBT3.3 in mediating activation of chromatin remodeling during priming induction was further evaluated in SBT3.3 defective plants. Figure 7D, F and G show that BTH-mediated increases of H3K4me3 and H3K9ac activation marks in the PR-1, WRKY6 and WRKY53 gene promoters was partially impeded in sbt3.3 plant when compared to Col-0 plants. For the SBT3.3 gene promoter (Fig. 7E), the reduction in histone activation marks following BTH treatment of sbt3.3 plants was most dramatic. This severe reduction was most notorious in the case of H3K4me3 activation marks for which a full inhibition was observed in BTH-treated sbt3.3 plants when compared to BTH-treated Col-0 plants (Figure 7D). All the above observations therefore imply that the extracellular SBT3.3 subtilase is an integral component mediating establishment of primed immunity, and moreover, it appears to be targeted for negative epigenetic control of this same mechanism.
Discussion
Priming, an evolutionarily conserved phenomenon where cells respond to much lower levels of a pathogenic stimulus in a more rapid and robust manner, is an important component of the various forms of IR described in mammals [8]–[10], plants [6], [18] and invertebrates [11]. Despite the importance of priming, the signal component(s) mediating this sensitized state remain elusive. The sensitized state is in part explained by presumably dormant or silent component characters, which accumulate during priming, and are required only after pathogenic challenge [18]. In this respect, pre-stress deposition of two MPK family members of signaling enzymes, MPK3 and MPK6, has been described to play an important role for priming in Arabidopsis [12]. However, it remains undetermined whether or not activation of additional factors operating upstream of the two MPKs is required to establish priming.
Reverse genetic analysis was applied to identify the Arabidospsis SBT3.3 gene, encoding a serine protease of the subtilisin clan, which is pivotal in control of a priming mechanism that leads to sensitization for activation of SA-dependent defense responses and IR. SBT3.3 overexpression in transgenic Arabidopsis plants elicited enhanced disease resistance to pathogens. However, this enhanced resistance was not preceded by a high constitutive expression of SA-responsive genes as occurs in different disease resistant mutants, which in general carry associated dwarfism, such as in cpr1, edr1 or csb3 [26, and references therein] to mention a few. Instead, the SBT3.3-mediated resistance can be explained by accelerated and heightened activation of SA-responsive genes, only elicited by pathogen inoculation, therefore mimicking a wild type plant activated for priming. This phenotype is blocked in a sid2 mutant background, which lacks SA, and is also blocked in the npr1 mutant affected in signaling downstream of SA. SBT3.3 thus functions as a positive regulator of innate immunity operating upstream of the SA pathway. Consistent with the gain-of-function phenotype, SBT3.3 suppression impairs induction of SA-responsive genes and causes enhanced susceptibility to infection by pathogens.
Interestingly, SBT3.3 expression is rapidly demanded during activation of innate immunity preceding the activation of SA-responsive genes. However, in contrast to PR genes, SBT3.3 expression does not require the SA pathway through the NPR1 regulator. Moreover, SBT3.3 activation responds very rapidly to H2O2, a common ROS species generated very early during PAMP recognition by PRR leading to activation of innate immune responses. Consistent with other early induced and SA-independent genes [46], H2O2 might be the first signal for early transcriptional reprogramming of SBT3.3. Congruently, Daudi et al. [47] showed that knocking down the Arabidopsis cell wall peroxidases PRX33/PRX34, required for apoplastic H2O2 generation during innate immune responses, leads to changes in the cell wall proteome with depletion of various PAMP-elicited proteins, among which SBT3.3 was conspicuous [48]. Localized SBT3.3 expression in the heterologous N. benthamiana system led to expression of the endogenous P69 homologue. Similarly, its stable expression in transgenic plants also led to activation of the endogenous SBT3.3 gene by what appears to be a self-induction mechanism, which adds further novelty to the SBT3.3 activation mode. We hypothesize that to a subsequent initial activation by a pathogen, the expressed SBT3.3 subtilase could initiate a signaling process, which would lead to its own expression, at least to a certain threshold level, as if forming a regulatory positive feedback loop circuit. Maintenance of this expression threshold level should be sufficient to keep cells in a sustained sensitized mode. The autonomous and sustained SBT3.3 expression pattern should consequently be the basis to explain the memory-based characteristics of priming and IR, manifested only secondarily in hosts after a primary infection. Thus, SBT3.3 appears key in regulating this type of training effect leading to IR. Interestingly, MPK3 and MPK6 activation, and to a lesser extend also MPK4/MPK11, were enhanced in SBT3.3OEX plants following infection with PsDC3000, and conversely, this activation was compromised in sbt3.3 plants. These results are congruent with increased OXI1 expression in SBT3.3OEX plants, a kinase required for MPK3 and MPK6 activation [33], and indicates that following SBT3.3 expression, plants respond faster to pathogenic stimuli. Because the MPK3 and MPK6 activation is critical for priming [12], and activation requires OXI1 expression [33], our data suggest SBT3.3 positively modulates immune responses upstream of the MAPK kinase pathway, and confirms that accumulation of defense signaling components, such as SBT3.3 itself, prior to a secondary pathogen challenge is essential for priming and induced resistance.
Similarly, the observation that sole SBT3.3 expression poises SA-responsive defense genes for enhanced activation following perception of a pathogenic cue provides further support to SBT3.3 as an integral component in mounting primed immunity. ChIP assays revealed this poising effect for enhanced gene expression triggered by SBT3.3 was mediated by selective increases of histone activation marks on the promoter region of SA-responsive genes. Since similar histone activation marks have been found to appear in SA–related genes when wild type plants are treated with the priming agent BTH [20], our data therefore strongly support a model where SBT3.3 positively mediates OXI1-mediated MPK activation, and concurrent chromatin remodeling at SA-responsive genes as specific hallmarks for primed immunity. Furthermore, the observation that immune priming, and similar chromatin remodeling of SA-responsive genes is mirrored in plants defective in the RdDM pathway [23] provides support for a hypothesis that the observed SBT3.3-mediated priming mechanism might be under similar epigenetic control. Moreover, the fact that SBT3.3 gene expression is constitutively up in the nrpd2 mutant favors this interpretation. Furthermore, (1) the observation that histone activation marks are established in the promoter of the SBT3.3 gene and in the promoters of SA-dependent genes by individual SBT3.3 overexpression in transgenic plants; (2) the reproducibility of similar chromatin remodeling of the SBT3.3 gene promoter in nrpd2 plants; (3) its similar remodeling in Col-0 plants following BTH treatment; (4) and the observation that such chromatin remodeling is strongly abrogated in sbt3.3 plants, further substantiates the importance of SBT3.3 gene activation as a prerequisite for establishment of immune priming.
How does then a proteolytic enzyme such as SBT3.3, which accumulates in the extracellular matrix trigger activation of such a complex signaling pathway mediating priming and IR? One simple explanation is via SBT3.3-mediated protein substrate processing that co-localizes extracellularly. This substrate could exist in a soluble form, or be an extracellular domain (ectodomain) of a larger protein, likely functioning as a receptor located in the plasma membrane. After proteolytic shedding of the ectodomain by SBT3.3, the receptor could become activated and initiate a down stream immune signaling process. This mechanism has been identified as common in activating a variety of signaling processes in animal through the involvement of protease-activated receptors (PARs), a group of receptors mediating different cellular processes including proinflammatory responses, and is also a common principle in various diseases [49]. Moreover, the proteolytic processing mechanism of an extracellular substrate is reminiscent of that mediating activation of innate immunity in invertebrates through the transmembrane Toll receptor, or Toll-like receptors (TLRs) in humans; PRR-type receptors consisting of a leucine-rich repeat (LRR) ectodomain, a transmembrane domain, and a cytosolic signaling domain [Toll/IL-1 receptor (TIR)], which becomes activated only after the binding of a proteolytically processed peptide ligand (i.e. spätzle) by complex cascades of CLIP-domain serine proteases [50] or less common following specific cleavage of the receptor ectodomain by extracellular proteases [51], [52]. Alternatively, SBT3.3 processes the extracellular substrate and the cleaved polypeptide can be released and function as a ligand recognized by a nearby specific extracellular receptor, which in turn can initiate a downstream signaling. Tornero et al., [30] reported SBT3.3 homologous P69C subtilase can specifically process LRP in disease tomato plants, an extracellular LRR-containing protein of unknown function, and the first subtilase substrate identified in plants. This suggesting SBT3.3 could similarly be involved in the cleavage and activation of LRR-containing proteins, including PRR-type receptors, which in turn may activate innate immune responses. The recent finding that the lectin receptor kinase (LecRK)-VI.2, a member of the LRR-containing superfamily of RLKs proteins existing in Arabidopsis, is required for immune priming acting upstream of MPK-mediated signaling [53] can give further support to this hypothesis.
The results of the present study identified SBT3.3 as a determinant host factor mediating activation of primed immune responses. Our immediate future challenge is to identify the target substrate processed by this subtilase and elucidate the mechanism transducing the substrate into a signal for immune prime activation.
Materials and Methods
Plants growth conditions
Arabidopsis thaliana and Nicotiana benthamiana plants were grown in a growth chamber (19–23°C, 85% relative humidity, 100 mEm−2 sec−1 fluorescent illumination) on a 10-hr-light and 14-hr-dark cycle. All mutants are in Col-0 background; nrpd2-2, npr1-1 and sid2-1 plants were previously described [23], [26], [54]. sbt3.3-1, sbt3.3-2, sbt3.4-1 and sbt3.4-2 mutants and SBT3.3OEX1 and SBT3.3OEX2 overexpression lines were obtained from the Plant Subtilase Database Consortium (PSDB) (http://csbdb.mpimp-golm.mpg.de/csbdb/dbcawp/psdb.html).
Gene constructs and transgenic lines
For the SBT3.3-GFP overexpressing construct, a full length cDNA for SBT3.3 was amplified by PCR using Pfu DNA polymerase (Stratagene, San Diego, CA) and specific primers including Gateway adapters: BP SBT3.3 FW and BP SBT3.3 RV and recombined into pDONR207 using BP ClonaseMixII kit (Invitrogen). For the SBT3.3m-GFP construct, pDONR207+SBT3.3 vector was amplified using Phusion Hot Start II polymerase (Thermo Scientific) with SBT3.3m FW and SBT3.3m RV phosphorylated primers including a T663 to G663 mutation. The PCR product was then digested with DpnI restriction enzyme (Fermentas), purified by Zymoclean DNA Recovery Kit (Zymo Research) and religated using T4 Ligase (Fermentas). For SBT3.3noSP-GFP construct pDONR207+SBT3.3 vector was amplified with SBT3.3noSP FW and SBT3.3 RV primers and recombined into pDONR207 as described above. After sequencing, all constructs were recombined with pB7FWG destination vector using LR ClonaseMixII kit (Invitrogen) and introduced into Arabidopsis (Col-0) via Agrobacterium transformation. The sid2-1 SBT3.3OEX and npr1-1 SBT3.3OEX lines were generated by the genetic crossing of the sid2-1 and npr1-1 mutants, respectively, with a 35S:SBT3.3 transgenic line containing a single insertion of the transgen. The sbt3.3 SBT3.3-GFP and sbt3.3 SBT3.3m-GFP lines were generated by direct genetic transformation of sbt3.3-1 plants with SBT3.3-GFP and SBT3.3m-GFP gene constructs, respectively. The selected lines were those expressing higher levels of the corresponding transgene as determined by RT-PCR of RNA preparations. For PCR-based detection of the sid2-1 mutant allele the primers used were sid2-1 Fw and sid2-1 Rv GCA GTC CGA AAG ACG ACC TCG AG and CTA TCG AAT GAT TCT AGA AGA AGC), followed by Mun I digestion of the ensuing fragment (the mutant allele sid2-1 cannot be digested). For PCR-based detection of the npr1-1 mutant allele, the primers used were npr1-1 Fw and npr1-1 Rv (5′-ATGTCTCGAATGTACATAAGGC-3′ and 5′-CTCAGTTTCCTAATAGAGAGG-3′).
Transient expression in Nicotiana benthamiana leaves
Almost fully expanded leaves were infiltrated with a suspension of Agrobacterium tumefaciens C58 bearing the relevant construct in 10 mM MES pH 5.6, 10 mM MgCl2, 150 µM acetosyringone at an OD600 = 0.5. After 3 days, fluorescence was analyzed in infiltrated leaves by confocal microscopy. For co-infiltration, Agrobacterium cultures grown separately and processed as indicated above, were adjusted to an O.D. = 0.5, and mixed prior to infiltration. Agrobacterium expressing the viral silencing suppressor P19 was included in all infiltrations.
Fluorescence microscopy
GFP/YFP fluorescence in inoculated plants was monitored using Nikon SMZ800, and Leica MZ16F microscopes.
Gene expression analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's recommendations and further purified by lithium chloride precipitation. For reverse transcription, the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas Life Sciences) was used. Quantitative PCR (qPCR) amplifications and measurements were performed using an ABI PRISM 7000 sequence detection system, and SYBR-Green (Perkin-Elmer Applied Biosystems). ACTIN2 was chosen as the reference gene. The primers used to amplify the different genes and DNA regions, and the PCR conditions employed for genotyping T-DNA insertions, and RT-PCR and qRT-PCR experiments are provided in the supporting information file Text S1. RT-qPCR analyses were performed at least three times using sets of cDNA samples from independent experiments.
Microarray hybridization and data analysis
Affymetrix microarrays (Arabidopsis ATH1 genome array) containing 22,810 probe sets were used. Labeling and hybridization on the ATH1 microarrays were performed according to the manufacturer's instructions (www.affymetrix.com/support/technical/manual/expression_manual.affx). Global analysis of gene expression was performed by using Affymetrix MAS5.0. SAM analysis (Significance Analysis of Microarrays software package) was conducted for A. thaliana triplicate samples between csb3 plants and control plants using a q value≤0.05 and a fold change cutoff ≥2 to identify the genes differentially expressed in the mutant. We searched GO enrichment information for the differently expressed probe sets using EasyGO (http://bioinformatics.cau.edu.cn/easygo/ category_treeBrowse.html). We applied χ 2 analysis for the biological process search, and the cutoff for false discovery rate (FDR) was adjusted using a p value of 0.0001. GeneChip data set are available in a MIAME-compliant format through GEO (accession no. GSE35507).
Bacterial and oomycete bioassays
Bacterial strains were grown overnight and used to infect 5-week-old Arabidopsis leaves by infiltration and bacterial growth determined following [23], [54]. Twelve samples were used for each data point and represented as the mean ± SEM of log c.f.u./cm2. H. arabidopsidis WACO9 sporangia were obtained by washing sporulating Col-0 leaves in 10 mM MgSO4, collected by centrifugation, and resuspended in 10 mM MgSO4 to a final density of 5×104 sporangia per mL as described [25]. Three-week-old seedlings were challenge inoculated with H. arabidopsidis by spraying with 10 mM MgSO4 containing 5×104 conidiospores per mL. Inoculated plants were maintained at 17°C and 100% relative humidity. Disease symptoms were scored for about 200 leaves per treatment at 7 days after challenge. For determining leaf colonization, infected leaves were stained with lactophenol trypan-blue and examined microscopically at 7 days after inoculation, as described [25] and scored on each leaf in the following classes: I, no colonization; II, low tissue colonization (<25% of leaf area colonized); III, medium tissue colonization (25–50% of leaf area colonized); IV, high tissue colonization (>50% of leaf area colonized). Sporulation was expressed as intensity of pathogen sporulation on each leaf: I, no sporulation; II, <50% of the leaf area covered by sporangiophores; III, >50% of the leaf area covered by sporangiophores; and IV, heavily covered with sporangiophores, with additional chlorosis and leaf collapse. When indicated, oomycete spore counting was performed as previously described [26].
Chromatin immunoprecipitation
Chromatin isolation and immunoprecipitation were performed as described [55]. Chip samples, derived from three biological replicates, were amplified in triplicate and measured by quantitative PCR using primers for PR-1, WRKY6, WRKY53 and Actin2 as reported [21]. The rest of primers are described in Text S1 file. All ChIP experiments were performed in three independent biological replicates. The antibodies used for immunoprecipitation of modified histones from 2 g of leaf material were antiH3K4m3 (#07-473 Millipore) and antiH3K4ac (#07-352 Millipore).
Western blot
Protein crude extracts were prepared by homogenizing ground frozen leaf material with Tris-buffered saline (TBS) supplemented with 5 mM DTT, protease inhibitor cocktail (Sigma-Aldrich), and protein phosphatase inhibitors (PhosStop, Roche). Protein concentration was measured using Bradford reagent; 25 µg of total protein was separated by SDS-PAGE (12% acrylamide w/v) and transferred to nitrocellulose filters. The filter was stained with Ponceau-S after transfer, and used as a loading control.
Supporting Information
Pie chart categorizing genes which are differentially expressed in Col-0 and csb3 plants. Genes with p-values less than 0,05 and fold changes greater than 2 are included. These genes are grouped based on their functional annotations and normed to frequency of class over the genome using Classification Superviewer (www.bar.utoronto.ca). Number of genes of each class is indicated.
(TIF)
Bootstrapped consensus neighbour-joining tree generated from an alignment of the annotated 56 AtSBT full-length protein sequences. Gene expression analysis of the 56 Arabidopsis subtilase members in response to SA, MeJA, ACC, ABA, P. syringae DC3000 (Ps), B. cinerea (Bc) and oxydative stress (OX). Response analyzed by microarray database analysis using the Botany Array Resource program (Toufighi et al., 2005). AtSBT3.3 (At1g32960) is highlighted in bold.
(TIF)
Deduced amino acid sequence of the gene encoding SBT3.3 subtilase. The catalytically important Asp, His, Asn, and Ser residues are in boldface typed in blue and indicated with asterisks. The propeptide domain in indicated in green. The signal peptide is indicated in red. Potential consensus sequences for N-glycosylation are marked in orange.
(TIF)
Comparative induction of the SA-dependent PR-1 gene and the SBT3.3 gene expression by application of 1 µM Fgl22. RT-qPCR analysis showing gene expression in mock- (white columns) and Fgl22-treated (solid columns) Col-0 seedlings 1 h after treatment. Data represent the mean ± SD; n = 3 biological replicates. Expression was normalized to the expression of the constitutive ACT2 gene and then to the expression in time 0 Col-0 plants.
(TIF)
Disease responses to H. arabidopsidis as assessed by direct counting of spore production on inoculated plants. To quantify resistance to H. arabidopsidis, production of spores was counted 7 days after inoculation. Plants carrying the sbt3.3 mutations were highly resistant to this pathogen while overexpression of SBT3.3 conferred enhanced resistance to this pathogen. Error bars represent standard deviation (n = 30). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Extracellular localization of SBT3.3-GFP in transgenic Arabidopsis leaves by confocal microscopy. Expression of SBT3.3-GFP in transgenic Arabidopsis results in a uniform extracellular fluorescence. Upper panel shows GFP localization in leaves of transgenic plants expressing SBT3.3-GFP. Lower panel shows a magnification of the tissue section shown in the upper panel.
(TIF)
Expression of a missense mutant of SBT3.3 (S555A; SBT3.3m) in N. benthamiana leaves no longer promotes accumulation of the endogenous P69 subtilase or PR-1a proteins. Total protein extracts from N. benthamiana leaves transitorily overexpressing GFP alone, SBT3.3-GFP or SBT3.3m-GFP fusion proteins were separated on a 10% SDS-PAGE gel, transferred to nitrocellulose and the blots revealed with anti-GFP antibodies (α-GFP; upper panels), anti-P69 antibodies (α-P69) and anti-PR-1a antibodies (α-PR-1a). Total protein extracts from empty A. tumefaciens agroinfiltrated N. benthamiana leaves (mock) were used as controls. The sizes of the marker proteins are indicated by arrows. Equal protein loading was monitored by staining the filters with Ponceau.
(TIF)
Transgenic 35S::SBT3.3-GFP plants, but not transgenic 35S::SBT3.3m-GFP plants, show enhanced disease resistance towards Ps DC3000. Col-0 plants were genetically transformed with 35S::SBT3.3-GFP and 35S::SBT3.3m-GFP and stable homozygous lines sowing expression of the transgene were selected for evaluation of the resistance phenotype towards PsDC3000 in comparison to Col-0 plants and SBT3.3OEX1 plants. Five-week-old plants of the indicated genetic backgrounds were inoculated with PsDC3000 and the bacterial growth measured at five days post-inoculation. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Transgenic sbt3.3 plants expressing SBT3.3-GFP lose the enhanced disease susceptibility to P. syringae DC3000. sbt3.3 and Col-0 plants were stably transformed with a 35S::SBT3.3-GFP construct and two independent stable homozygous lines sowing expression of the transgene were selected for evaluation of the resistance phenotype towards PsDC3000 in comparison to untransformed plants. Five-week-old plants of the indicated genetic backgrounds were inoculated with PsDC3000 and the bacterial growth measured at five days post-inoculation. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Genes up and down regulated in the csb3 mutant.
(XLSX)
Defense-related genes up-regulated (≥2 fold) in the Arabidopsis csb3 mutant with respect to wild type (wt) plants. AtSBT3.3 (At1g32960) is highlighted in bold.
(TIF)
Primer sequences.
(XLSX)
Acknowledgments
We thank the Plant Subtilase Database (PSDB) for providing some of the Arabidopsis transgenic lines overexpressing SBT3.3 that are described in this work.
Funding Statement
The Spanish MICINN (BFU2009-09771, EUI2009-04009 to PV) and Generalitat Valenciana (Prometeo2010/020 to PV) provided support for this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 2. Moore JW, Loake GJ, Spoel SH (2011) Transcription dynamics in plant immunity. Plant Cell 23: 2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Netea MG, Quintin J, van der Meer JWM (2011) Trained immunity: a memory for innate host defese. Cell Host & Microbe 9: 355–361. [DOI] [PubMed] [Google Scholar]
- 4. Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209. [DOI] [PubMed] [Google Scholar]
- 5. Van Wees SCM, Van der Ent S, Pieterse CMJ (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11: 443–448. [DOI] [PubMed] [Google Scholar]
- 6. Kuć J (1987) Translocated signals for plant immunization. Ann N Y Acad. Sci 494: 221–223. [Google Scholar]
- 7. Zimmerli L, Jakab G, Métraux J-P, Mauch-Mani B (2000) Potentiation of pathogen-specific defense mechanisms in Arabidopsis by β-aminobutyric acid. Proc Natl Acad Sci USA 97: 12920–12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes MP, Enterline JC, Gerrard TL, Zoon KC (1991) Regulation of interferon production by human monocytes: requirements for priming for lipopolysaccharide-induced production. J Leukoc Biol 50: 176–181. [DOI] [PubMed] [Google Scholar]
- 9. Gifford GE, Lohmann-Matthes M-L (1987) Gamma interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J Natl Cancer Inst 78: 121–124. [DOI] [PubMed] [Google Scholar]
- 10. Koerner TJ, Adams DO, Hamilton T (1987) Regulation of tumor necrosis factor (TNF) expression: Interferon-γ enhances the accumulation of mRNA for TNF induced by lipopolysaccharide in murine peritoneal macrophages. Cell Immunol 109: 437–443. [DOI] [PubMed] [Google Scholar]
- 11. Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS (2007) A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog 3: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beckers GJM, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, et al. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana . Plant Cell 21: 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohler A, Schwindling S, Conrath U (2002) Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis . Plant Physiol 128: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dempsey D'MA, Klessig DF (2012) SOS-too many signals for systemic acquired resistance? Trends Plant Sci 17: 538–545. [DOI] [PubMed] [Google Scholar]
- 15. Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324: 89–91. [DOI] [PubMed] [Google Scholar]
- 16. Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, et al. (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zimmerli L, Métraux J-P, Mauch-Mani B (2000) Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinérea . Plant Physiol 126: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prime-A-Plant Group: Conrath, U., (2006) et al. Priming: getting ready for battle. Mol Plant-Microbe Interact 19: 1062–1071. [DOI] [PubMed] [Google Scholar]
- 19. Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nature Rev Immunol 12: 89–100. [DOI] [PubMed] [Google Scholar]
- 20. Jaskiewicz M, Conrath U, Peterhänsel C (2011) Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosher RA, Durrant WE, Wang D, Song J, Dong X (2006) A comprehensive structure–function analysis of Arabidopsis SNI1 defines essential regions and transcriptional repressor activity. Plant Cell 18: 1750–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Law JA, Jacobsen SE (2009) Dynamic DNA methylation. Science 323: 1568–1569. [DOI] [PubMed] [Google Scholar]
- 23. López A, Ramirez V, Garcia-Andrade J, Flors V, Vera P (2011) The RNA Polymerase V Is Required for Plant Immunity. PLoS Genet 7 (12) e1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luna E, Bruce TJA, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slaughter A, Daniel X, Flors V, Luna E, Hohn B, et al. (2012) Descendants of primed Arabidopsis plants exhibit enhanced resistance to biotic stress. Plant Physiol 158: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gil MJ, Coego A, Mauch-Mani B, Jordá L, Vera P (2005) The Arabidopsis csb3 mutant reveals a regulatory link between salicylic acid-mediated disease resistance and the methyl-erythritol 4-phosphate pathway. Plant Journal 44: 155–166. [DOI] [PubMed] [Google Scholar]
- 27. Jorda L, Vera P (2000) Local and systemic induction of two defense-related subtilisin-like protease promoters in transgenic Arabidopsis plants. Plant Physiol 124: 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rautengarten C, Steinhauser D, Büssis D, Stintzi A, Schaller A, et al. (2005) Inferring Hypotheses on Functional Relationships of Genes: Analysis of the Arabidopsis thaliana Subtilase Gene Family. PLoS Comput Biol 1 (4) e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jorda L, Coego A, Vera P (1999) A genomic cluster containing four differentially regulated subtilisin-like processing protease genes is in tomato plants. J Biol Chem 274: 2360–2365. [DOI] [PubMed] [Google Scholar]
- 30. Tornero P, Conejero V, Vera P (1996) Primary structure and expression of a pathogen-induced protease (P69) in tomato plants: Similarity of functional domains to subtilisin-like endoproteases. Proc Natl Acad Sci USA 93: 6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong X, Mindrinos M, Davis KR, Ausubel FM (1991) Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3: 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolwell GP, Daudi A (2009) Reactive oxygen species in plant-pathogen interactions. In LA del Rio, A Puppo, eds, Reactive Oxygen Species in Plant Signaling. Springer-Verlag, Berlin, pp 113–133. [Google Scholar]
- 33. Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, et al. (2004) OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis . Nature 427: 858–861. [DOI] [PubMed] [Google Scholar]
- 34. Schaller A, Stintzi A, Graff L (2012) Subtilases - versatile tools for protein turnover, plant development, and interactions with the environment. Physiol Plant 145: 52–66. [DOI] [PubMed] [Google Scholar]
- 35. Takeda N, Sato S, Asamizu E, Tabata S, Parniske M (2009) Apoplastic plant subtilases support arbuscular mycorrhiza development in Lotus japonicus. Plant J 58: 766–777. [DOI] [PubMed] [Google Scholar]
- 36. Chichkova NV, Shaw J, Galiullina RA, Drury GE, Tuzhikov AI, et al. (2010) Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. EMBO J 29: 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bykova NV, Rampitsch C, Krokhin O, Standing KG, Ens W (2006) Determination and characterization of site-specific N-glycosylation using MALDI-Qq-TOF tandem mas spectrometry: case study with a plant protease. Anal Chem 78: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 38. Cedzich A, Huttenlocher F, Kuhn BM, Pfannstiel J, Gabler L, et al. (2009) The protease-associated domain and C-terminal extension are required for zymogen processing, sorting within the secretory pathway, and activity of tomato subtilase 3 (SlSBT3). J Biol Chem 284: 14068–14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–83. [DOI] [PubMed] [Google Scholar]
- 41. Bethke G, Unthan T, Uhrig JF, Pöschl Y, Gust AA, et al. (2009) Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci USA 106: 8067–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bethke G, Pecher P, Eschen-Lippold L, Tesuda K, Katagiri F, et al. (2012) Activation of the Arabidopsis thaliana mitogen-activated protein kinase MPK11 by the flagellin-derived elicitor peptide, flg22. Mol Plant-Microbe Interactions 25: 471–480. [DOI] [PubMed] [Google Scholar]
- 43. Rushton PJ, Somssich IE, Ringler P, Shen QJ (2010) WRKY transcription factors. Trends Plant Sci 15: 247–258. [DOI] [PubMed] [Google Scholar]
- 44. Dong J, Chen C, Chen Z (2003) Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol 51: 21–37. [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Deng X, Miki D, Cutler S, La H, Hou Y-J, et al. (2012) Sulfamethazine suppresses epigenetic silencing in Arabidopsis by impairing folate synthesis. Plant Cell 24: 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coego A, Ramírez V, Ellul P, Mayda E, Vera P (2005) The H2O2-regulated Ep5C gene encodes a peroxidase required for bacterial speck susceptibility in tomato. Plant J 42: 283–293. [DOI] [PubMed] [Google Scholar]
- 47. Daudi A, Cheng Z, O'Brien JA, Mammarella N, Khan S, et al. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. O'Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, et al. (2012) A Peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells Functions in MAMP-elicited defense. Plant Physiol 158: 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ossovskaya VS, Bunnett NW (2004) Protease-activated receptors: contribution to physiology and disease. Physiol Rev 84: 579–621. [DOI] [PubMed] [Google Scholar]
- 50. Buchon N, Poidevin M, Kwon H-M, Guillou A, Sottas V, et al. (2009) A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci USA 106: 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brunn GJ, Bungum MK, Johnson GB, Platt JL (2005) Conditional signaling by Toll-like receptor 4. FASEB J 19: 872–874. [DOI] [PubMed] [Google Scholar]
- 52. de Zoete MR, Bouwman LI, Keestra AM, van Putten JPM (2011) Cleavage and activation of a Toll-like receptor by microbial proteases. Proc Natl Acad Sci USA 108: 4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh P, Kuo Y-C, Mishra S, Tsai C-H, Chien C-C, et al. (2012) The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24: 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agorio A, Vera P (2007) ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 19: 3778–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haring M, Offermann S, Danker T, Horst I, Peterhansel C, et al. (2007) Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pie chart categorizing genes which are differentially expressed in Col-0 and csb3 plants. Genes with p-values less than 0,05 and fold changes greater than 2 are included. These genes are grouped based on their functional annotations and normed to frequency of class over the genome using Classification Superviewer (www.bar.utoronto.ca). Number of genes of each class is indicated.
(TIF)
Bootstrapped consensus neighbour-joining tree generated from an alignment of the annotated 56 AtSBT full-length protein sequences. Gene expression analysis of the 56 Arabidopsis subtilase members in response to SA, MeJA, ACC, ABA, P. syringae DC3000 (Ps), B. cinerea (Bc) and oxydative stress (OX). Response analyzed by microarray database analysis using the Botany Array Resource program (Toufighi et al., 2005). AtSBT3.3 (At1g32960) is highlighted in bold.
(TIF)
Deduced amino acid sequence of the gene encoding SBT3.3 subtilase. The catalytically important Asp, His, Asn, and Ser residues are in boldface typed in blue and indicated with asterisks. The propeptide domain in indicated in green. The signal peptide is indicated in red. Potential consensus sequences for N-glycosylation are marked in orange.
(TIF)
Comparative induction of the SA-dependent PR-1 gene and the SBT3.3 gene expression by application of 1 µM Fgl22. RT-qPCR analysis showing gene expression in mock- (white columns) and Fgl22-treated (solid columns) Col-0 seedlings 1 h after treatment. Data represent the mean ± SD; n = 3 biological replicates. Expression was normalized to the expression of the constitutive ACT2 gene and then to the expression in time 0 Col-0 plants.
(TIF)
Disease responses to H. arabidopsidis as assessed by direct counting of spore production on inoculated plants. To quantify resistance to H. arabidopsidis, production of spores was counted 7 days after inoculation. Plants carrying the sbt3.3 mutations were highly resistant to this pathogen while overexpression of SBT3.3 conferred enhanced resistance to this pathogen. Error bars represent standard deviation (n = 30). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Extracellular localization of SBT3.3-GFP in transgenic Arabidopsis leaves by confocal microscopy. Expression of SBT3.3-GFP in transgenic Arabidopsis results in a uniform extracellular fluorescence. Upper panel shows GFP localization in leaves of transgenic plants expressing SBT3.3-GFP. Lower panel shows a magnification of the tissue section shown in the upper panel.
(TIF)
Expression of a missense mutant of SBT3.3 (S555A; SBT3.3m) in N. benthamiana leaves no longer promotes accumulation of the endogenous P69 subtilase or PR-1a proteins. Total protein extracts from N. benthamiana leaves transitorily overexpressing GFP alone, SBT3.3-GFP or SBT3.3m-GFP fusion proteins were separated on a 10% SDS-PAGE gel, transferred to nitrocellulose and the blots revealed with anti-GFP antibodies (α-GFP; upper panels), anti-P69 antibodies (α-P69) and anti-PR-1a antibodies (α-PR-1a). Total protein extracts from empty A. tumefaciens agroinfiltrated N. benthamiana leaves (mock) were used as controls. The sizes of the marker proteins are indicated by arrows. Equal protein loading was monitored by staining the filters with Ponceau.
(TIF)
Transgenic 35S::SBT3.3-GFP plants, but not transgenic 35S::SBT3.3m-GFP plants, show enhanced disease resistance towards Ps DC3000. Col-0 plants were genetically transformed with 35S::SBT3.3-GFP and 35S::SBT3.3m-GFP and stable homozygous lines sowing expression of the transgene were selected for evaluation of the resistance phenotype towards PsDC3000 in comparison to Col-0 plants and SBT3.3OEX1 plants. Five-week-old plants of the indicated genetic backgrounds were inoculated with PsDC3000 and the bacterial growth measured at five days post-inoculation. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Transgenic sbt3.3 plants expressing SBT3.3-GFP lose the enhanced disease susceptibility to P. syringae DC3000. sbt3.3 and Col-0 plants were stably transformed with a 35S::SBT3.3-GFP construct and two independent stable homozygous lines sowing expression of the transgene were selected for evaluation of the resistance phenotype towards PsDC3000 in comparison to untransformed plants. Five-week-old plants of the indicated genetic backgrounds were inoculated with PsDC3000 and the bacterial growth measured at five days post-inoculation. Error bars represent standard deviation (n = 12). Asterisks indicate statistical differences to Col-0 (P<0.05) using Student's t test.
(TIF)
Genes up and down regulated in the csb3 mutant.
(XLSX)
Defense-related genes up-regulated (≥2 fold) in the Arabidopsis csb3 mutant with respect to wild type (wt) plants. AtSBT3.3 (At1g32960) is highlighted in bold.
(TIF)
Primer sequences.
(XLSX)