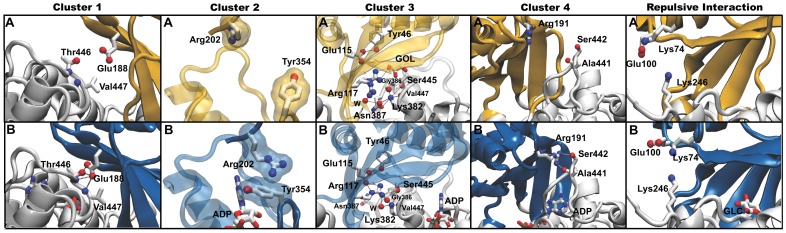
Figure 7. Interactions clusters between the small and large domain that mediate the transition from the open to the closed conformation.
(A) Structure of the enzyme in the apo-form and (B) structure of the ternary TlGK·Mg·ADPβS·D-glucose complex. The small domain was colored in yellow (apo-enzyme) or blue (ternary complex) for clarity purposes. Side chains of residues are represented as sticks. The atoms involved in H-bonds are represented as spheres. The nucleotide (ADP), the cosolvent, glycerol (GOL) and water (W) are also shown. Cluster 1 achieves communication between the small and large domain through Glu188, Thr446 and Val447. Cluster 2 residues contribute to stabilize the ADP-induced conformational change. In the apo-enzyme, Arg202 of the large domain is over the active-site pocket and the side chain of Tyr354 is oriented toward the outside of the pocket. In contrast, in the TlGK·Mg·ADPβS·D-glucose ternary complex Arg202 and Tyr354 form a stacking interaction afforded by the rotation of their side-chains so as to allow a cation-π interaction. Cluster 3′s interaction network is rearranged upon ADP binding through the formation of H-bonds between the large and small domain that generates a net attraction. In cluster 4, Arg191 from the small domain H-bonds Ala441 and Ser442 from the small domain. The only net repulsive interaction is provided by cluster 5, involving Lys74 and Lys246; in the apo-enzyme Glu100 is H-bonded to Lys74 thus neutralizing its net charge, but in the ternary complex this stabilizing interaction is absent and Lys74 comes closer to Lys246 despite the associated unfavorable energy barrier.