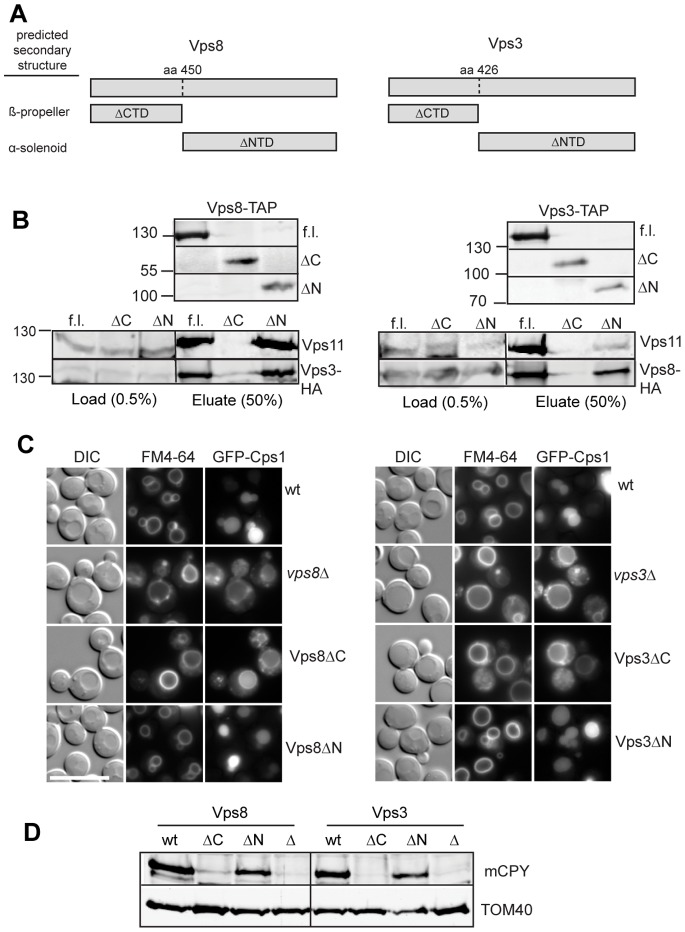
Figure 1. Consequences of domain deletions on CORVET functionality.
(A) Domain organization of the CORVET-specific subunits Vps8 and Vps3. N-terminal domains (NTDs/ΔCTDs) correspond to putative β-propeller, whereas C-terminal domains (CTDs/ΔNTDs) represent predicted α-solenoids segments. Domain boundaries were determined by the PredictProtein algorithm (www.predictprotein.org). (B) Purification of truncated Vps3 and Vps8 constructs. Small scale tandem affinity purification (TAP) of Vps8 and Vps3 fragments was carried out via IgG Sepharose. Glycin pH 2.5 - eluates were subjected to SDS-PAGE and Western-Blot via the Odyssey scanning system. Immunoprecipitated CORVET subunits were detected by antibodies against Cbp, Vps11 and HA. Expression of truncated constructs was confirmed by decoration eluates of the pull-down with anti-Cbp antibodies (top panel). Sizes are indicated in kDa. (C) Vacuole morphology and endocytic sorting in Vps3 and Vps8 mutants. Sorting of N-terminally GFP-tagged Cps1, expressed from CEN-plasmids, was detected by fluorescence microscopy. To monitor vacuole-morphology, cells were stained with FM4–64. Size bar, 10 µm. See methods for details. (D) Transport of carboxypeptidase Y (CPY). Sorting was monitored by detection of processed CPY (mCPY) from vacuole enriched pellet fractions. Absence of mCPY is due to secretion and defective vacuolar protein sorting. Blots were decorated against Tom40 as loading control.