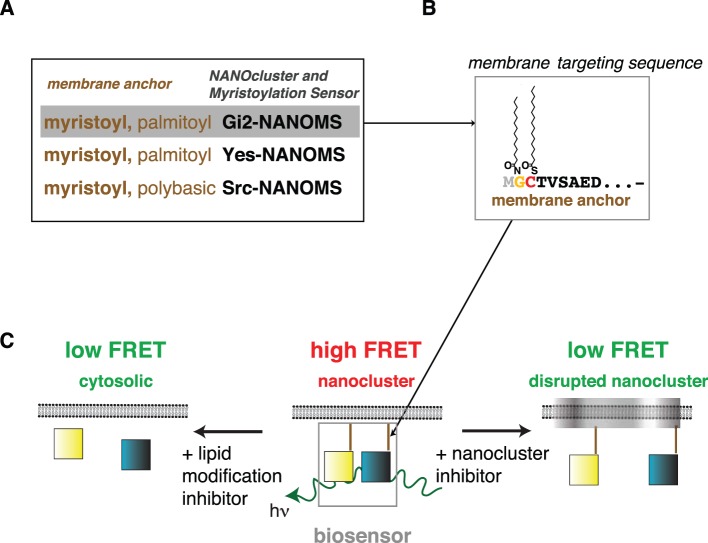
Figure 1. Design and reporting principle of NANOMS.
FRET-biosensor design of the three different NANOMS. (A) The myristoylated N-terminal membrane-targeting motifs of mouse Gαi2 (residues 1–35), human Yes (1–17)- and human Src (1–16)-kinases were genetically fused to the N-terminus of fluorescent proteins mCFP or mCit. The sequence of the employed membrane-targeting motifs can be found in Table S2. (B) Intracellular processing involves cleavage of the N-terminal methionine (grey) by methionine amino-peptidase (Met-AP), NMT-mediated myristoylation on glycine 2 (yellow) and depending on the motif cysteine-palmitoylation (red). (C) Lipid modified reporters spontaneously organize into plasma membrane nanocluster. Tight packing of membrane targeted donor (mCFP)- and acceptor (mCit)-fluorophores (blue and yellow squares, respectively) in nanocluster leads to FRET. FRET can decrease due to loss of nanoclustering or cytoplasmic redistribution of the NANOMS after inhibitor treatment. As membrane anchorage is required for the functioning of myristoylated proteins, NANOMS report on functional membrane anchorage.