Abstract
We demonstrate that decursin induces apoptosis via regulation of cyclooxygenase-2 (COX-2) and survivin in leukemic KBM-5 cells. By activating an apoptotic machinery, decursin is cytotoxic to KBM-5 cells. In this apoptotic process, decursin can activate caspase family members and triggers PARP cleavage. At the same time, the expression of COX-2 and survivin in the cells is downregulated. Furthermore, decursin is in synergy with COX-2 inhibitor, celecoxib or NS398 for the induction of apoptosis. Overall, these results suggest that decursin, via inhibiting COX-2 and survivin, sensitizes human leukemia cells to apoptosis and is a potential chemotherapeutic agent to treat this disease.
Keywords: Decursin, Apoptosis, COX-2, Survivin, KBM-5
1. Introduction
Chronic myelogenous leukemia (CML) is a form of leukemia characterized by abnormal proliferation of myeloid cells in the bone marrow [1]. Mitogenic signaling pathways in CML cells has been shown to be in hyperactive status mainitained by constitutively activated protein tyrosine kinase p210 BCR-ABL[2]. Targeted therapy, such as using Gleveec (a specific inhibitor of this kinase) is clinically approved for CML and has been shown to be successful. Nonetheless, the resistance and toxicity of these anti-CML drugs remain problematic. In addition, these drugs often have serious side effects include nausea, fluid retention and diarrhea. Natural compounds are generally believed to be associated with little toxicity and less drug resistance for CML treatment.
Angelica gigas Nakai (Umbelliferae) root has been used for the prevention and treatment of anemia as a tonic agent [3]. Decursin, a major compound of Angelica gigas [4], was reported to have anti-tumor activity against various types of cancer cells through inducing st or apoptosis [5-9]. However, the underlying molecular mechanisms of decursin with respect to its action against CML are not fully understood yet. In the current study, we demonstrated that decursin, via regulating COX-2 and survivin, achieves its anticancer function.
2. Materials and methods
2.1. Natural chemical
Decursin (Fig. 1A) was extracted and purified as described previously [10].
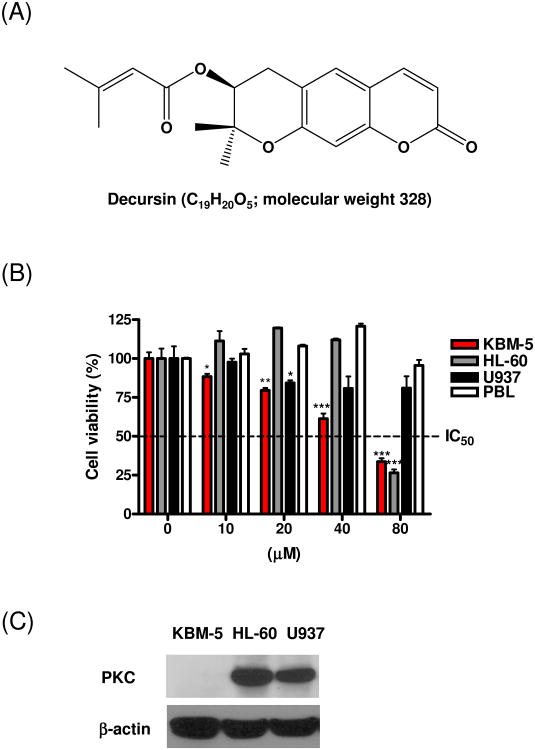
Fig. 1.
Cytotoxic effect of decursin against human leukemic cells. (A) Chemical structure of decursin (MW. 328). (B) Human leukemia KBM-5, HL-60 and U937 cells and human peripheral blood lymphocyte (PBL) cells were incubated with various concentrations of decursin for 24 h. Cell viability was determined by the XTT assay. The data represent mean ± S.D. Statistically significant difference was calculated by Student's t-test. *, p < 0.05, **, p < 0.01 and **, p < 0.001 versus control. (C) Western blotting was performed using cell lysates from human leukemia KBM-5, HL-60 and U937 cells with anti-PKC antibody.
2.2 Cell culture
Human leukemia KBM-5 cells were cultured in Iscove's modified Dulbecco's media (IMDM) (Welgene, Deagu, South Korea) supplemented with 15% fetal bovine serum (FBS) and penicillin/streptomycin. Human promyelocytic leukemia HL-60 and human leukemic monocyte lymphoma U937 cells were cultured in RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin. All cells were maintained at 37°C in a humidified atmosphere containing 5 % CO2.
2.3. Peripheral blood lymphocyte (PBL) isolation
PBL was isolated from 20 ml of blood samples from healthy human donor by Ficoll-Hypaque (GE Health Care Bio-Sciences, Piscataway, NJ) gradient centrifugation.
2.4. Cytotoxicity assay
The cytotoxicity of decursin was measured using XTT colorimetric assays. Cells were seeded into 96-well microplates at a density of 2 × 104 cells per well in 100 μl of growth medium with various concentrations of decursin (10, 20, 40 or 80 μM), and incubated at 37°C in a humidified incubator containing 5 % CO2 for 24 h. XTT working solution was prepared by mixing 1 ml of XTT stock solution (1 mg/ml in PBS) with 10 μl of phenazine methosulphate (PMS) [1.53 mg/ml in phosphate buffered saline (PBS)], 50 μl of XTT working solution freshly prepared was added to each well. Cells were incubated at 37°C for 2 h and the optical de was measured using a microplate reader (Sunrise, TECAN, Männedorf, Switzerland) at 450 nm. Cell viability was calculated as a percentage of viable cells in decursin-treated group versus PBS-treated control by the following equation. Cell viability (%) = [OD (Decursin) – OD (Blank)/OD (Control) – OD (Blank)] × 100.
2.5. Live and Dead assay
To measure apoptosis, we used the Live and Dead assay kit (Molecular Probes, Carlsbad, CA), which determines intracellular esterase activity and plasma membrane integrity. This assay uses calcein, a polyanionic dye, which is retained in live cells and provides green fluorescence. It also uses the ethidium monomer dye (red fluorescence), which can enter cells only through damaged membranes and bind to nucleic acids but is excluded by the intact plasma membrane of live cells. In brief, 1 × 106 cells were incubated with decursin for 24 h, then stained with the Live and Dead reagent (5 μM ethidium homodimer and 5 μM calcein-AM) and incubated at 37°C for 30 min. The cells were analyzed under an Axio vision 4.0 fluorescence microscope (Carl Zeiss Inc., Weimar, Germany).
2.6. TUNEL assay
Flow cytometric analysis was carried out using an in situ cell death detection reagent (Roche Molecular Biochemicals, Mannheim, Germany) as described by the manufacturer's instructions. Labeling of DNA strand breaks with fluorescein allows quantitative analysis of apoptosis by flow cytometry. DNA end (3′-OH) generated by DNA fragmentation was nick end-labeled with FITC-conjugated dUTP, introduced by terminal deoxytransferase (TdT) using an TUNEL label Mix (Roche Molecular Biochemicals, Mannheim, Germany) and TUNEL enzyme (Roche Molecular Biochemicals, Mannheim, Germany) and analyzed by flow cytometry. KBM-5 (1 × 106 cells) were incubated with various concentration of decursin for 24 h at 37°C. The cells were fixed in 4% paraformaldehyde in PBS at room temperature for 1 h, then washed in PBS and suspended in 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. The cells were washed twice in PBS and resuspended in reaction mixture with TUNEL enzyme and incubated for 1 h at 37°C in a humidified atmosphere in the dark. The cells were washed three times with PBS and analyzed by FACSCalibur flow cytometry (BD Biosciences, San Jose, CA).
2.7. Cell cycle analysis
To determine apoptosis, cell cycle analysis was performed as previously described (Herrmann et al., 1994). Cells (1 × 106 cells) treated with decursin were harvested, washed twice with cold PBS and fixed in 75% ethanol at -20°C. Fixed cells were resuspended in 100 μl of PBS containing 10 μl of RNase A (10 mg/ml) and incubated for 1 h at 37°C. The cells were added to 400 μl propidium iodide (50 μg/ml) and stained for 30 min at room temperature in the dark. The DNA contents of stained cells were analyzed using CellQuest Software with the FACSCalibur flow cytometry (BD Biosciences, San Jose, CA).
2.8. Western blot analysis
Cell lysates were prepared on ice in lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and 1X protease inhibitor cocktail. The lysates were spun at 14,000 × g for 20 min at 4°C and the supernatants were collected. Protein concentrations were determined by electrophoresis on 4-12% NuPAGE Bis-Tris gels (Novex, Carlsbad, CA). The proteins were then transferred to a Hybond ECL transfer membrane for detection by antibodies including anti-PARP, cleaved caspase-9, cleaved caspase-3, survivin, c-IAP1, c-IAP2, Bcl-2, Bcl-XL (Santa Cruz Biotechnologies, Santa Cruz, CA), COX-2 (Cayman Chemical, Ann Arbor, MI) and (β-actin (Sigma, St. Louis, MO).
2.9. Transfection
KBM-5 cells were transiently transfected with empty vector or pSPORT6-COX2 DNA (Invitrogen, Carlsbad, CA) for 48 h using Lipofectamine Plus transfection reagent (Invitrogen, Carlsbad, CA). The effects of decursin (80 μM) on the expression of COX- 2, survivin and cleaved caspase-3 were evaluated in wildtype and COX-2 overexpressed KBM-5 cells.
2.10. Immunofluorescence assay
For immunostaining, KBM-5 cells were fixed on poly-L-lysine coated slide in 4% paraformaldehyde and permeabilized in cold methanol. The permeabilized cells were then incubated with 10% normal goat serum in PBS for 1 hour, followed by immunostaining with mouse monoclonal anti-COX-2 (Becton Dickinson, Franklin Lakes, NJ), survivin (Santa Cruz Biotechnologies, Santa Cruz, CA), and rabbit polyclonal cleaved caspase-3 (Cell Signaling, Danvers, MA) antibodies. Mouse IgG FITC or rabbit IgG Texas Red antibody H & L (Abcam, Cambridge, MA) was used as the secondary antibody. The immunostained cells were mounted in medium containing DAPI (Vectashield, Vector Labs, Burlingame, CA) and visualized under a Carl Zeiss LSM5 confocal microscope.
2.11. Statistical analysis
All data were expressed as means ± SD. The statistically significant differences between decursin treated group and control group were calculated by Student's t test.
3. Results
3.1. Decursin is toxic to leukemia cells, but not to human peripheral blood lymphocytes
To analyze the cytotoxic effect of decursin on leukemia cells, human myeloid leukemia KBM-5 cells, promyelocytic leukemia HL-60 cells, leukemic monocyte lymphoma U937 cells and human peripheral blood lymphocytes (PBL) were treated with dimethyl sulfoxide (DMSO) or various concentrations (10, 20, 40 or 80 μM) of decursin for 24 h and subsequently cell viability was analyzed by XTT assay. Decursin started to be cytotoxic in KBM-5 cells at the concentration of 10 μM and its cytoxicity was in a dose-dependent fashion (Fig. 1B). Decursin showed its cytotoxicity against U937 cells at 20 μM, which became prominent at 80 μM. For HL-60 cells, decursin at 80 μM had an apoptotic effect. Decursin is known as a protein kinase C (PKC) modulator in cancer cells, especially leukemic cells [5,7,11]. To address the different cytotoxicity of decursin in the leukemia cells, PKC expression was analyzed by Western blotting. As shown in Fig. 1C, KBM-5 cells did not express PKC, while the kinase was present in HL-60 and U937 cells. These results imply that the different cytotoxicities of the cells to decursin may be due to different expression levels of PKC cells. Decursin did not show cytotoxicity against PBL at the concentration of up to 80 μM. These data suggested that decursin preferred killing KBM-5 cells rather than PBL cells.
3.2. Decursin induced apoptosis in KBM-5 cells
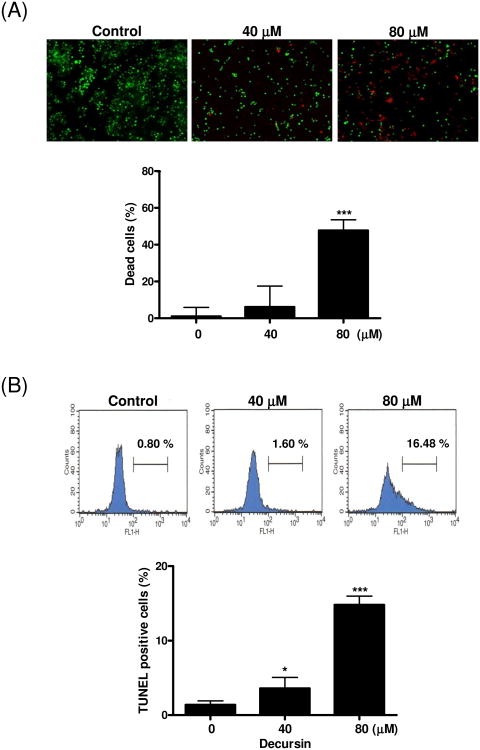
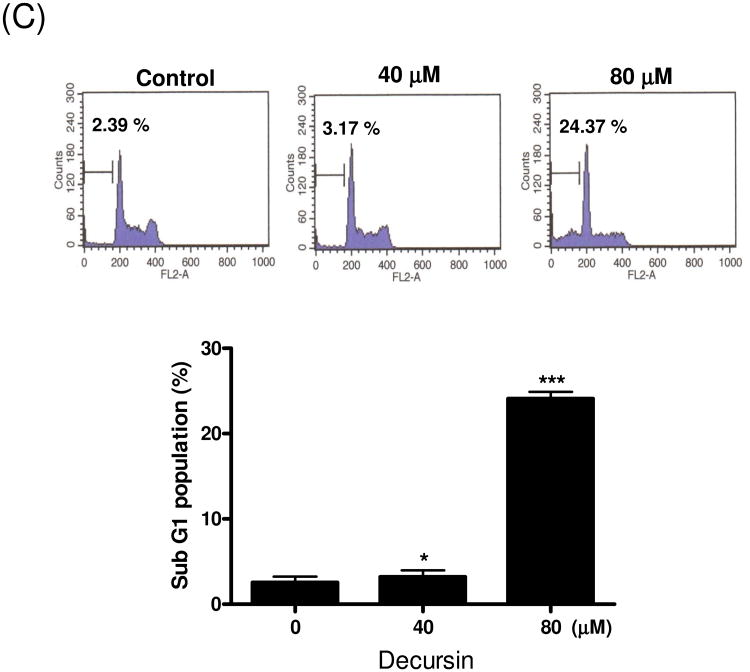
To further assess the cytotoxicity of decursin in KBM-5 cells, we performed the Live and Dead, TUNEL and cell cycle analyses. Decursin at 80 μM significantly increased the percentage of cell death (47.8%) (Fig. 2A). Consistently, TUNEL assay revealed a dramatic increase in TUNEL positive stained KBM5 cells (16.48%) after the treatment with decursin at 80 μM (Fig. 2B). In comparison, a few control cells (0.80%) underwent apoptosis in response to the same treatment. Consistently, decursin increased the apoptotic sub-G1 population of KBM-5 cells after the treatment with 80 μM of decursin (Fig. 2C).
Fig. 2.
Decursin-induced apoptosis in KBM-5 cells. Cells were treated with 0, 40 or 80 μM decursin for 24 h and subjected to apoptosis assays. (A) Cell death was determined by the calcein-AM-based Live/Dead assay as described in Materials and methods. Red highlights dead cells, and green highlights live cells. Percent of dead cells was represented. (B) Cells were labeled with TdT-mediated dUTP nick end labeling (TUNEL) and analyzed by flow cytometry. (C) For cell cycle analysis, decursin-treated cells were stained with PI after fixing in 75% ethanol. DNA contents of sub-G1 were analyzed by flow cytometry. The data represent mean ± S.D. Statistically significant difference was calculated by Student's t-test. *, p < 0.05, **, p < 0.01 and ***, p < 0.001 versus control.
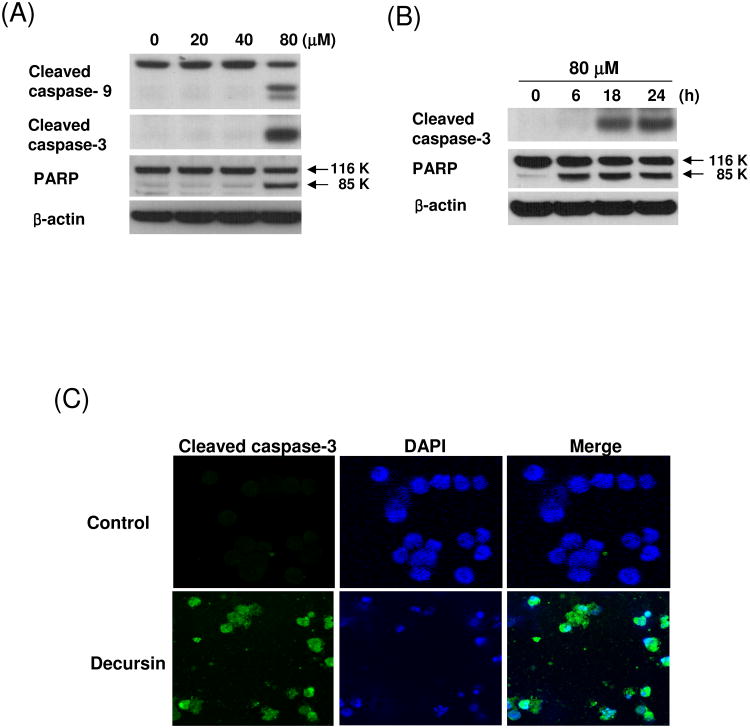
3.3. Decursin activates caspase 9, 3 and PARP in KBM-5 cells
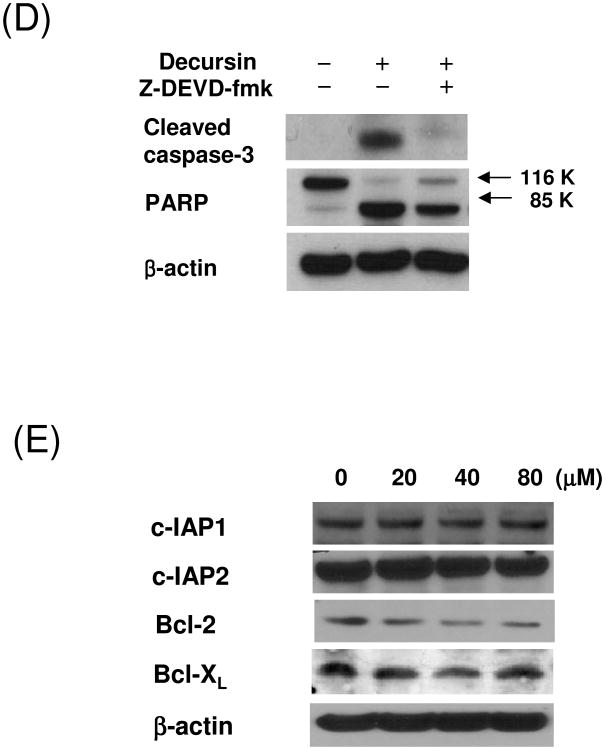
Caspases plays a central role in the induction of apoptosis [12]. To determine whether caspases were involved in decursin-induced apoptosis in KBM-5 cells, Western blotting analysis was performed. The treatment with decursin induced PARP cleavage, and the activation of caspase-9 and -3 in a concentration or time dependent manner (Fig. 3A and B). Immunofluorescence assay also showed the caspase-3 activation by decursin in KBM-5 cells (Fig. 3C). In addition, DAPI staining exhibited morphologically apoptotic features such as apoptotic bodies, cell shrinkage and nuclear condensation in decursin-treated cells. However, the addition of z-DEVD-fmk (a caspase-3 inhibitor) suppressed decursin-induced caspase-3 activation and PARP cleavage in KBM-5 cells, indicating that caspase-3 plays an important role in this apoptotic process (Fig 3D). Furthermore, decursin attenuated the expression of antiapoptotic proteins such as Bcl-2 and survivin, while it did not effectively affect expression of inhibitors of apoptosis protein (IAP) 1 and 2, and Bcl-XL in KBM-5 cells (Fig. 3E).
Fig. 3.
Caspase-3 activation in decursin-induced apoptosis in KBM-5 cells. (A) Cells were treated with 0, 20, 40 or 80 μM decursin for 24 h and Western blotting was performed for cleaved caspase-3, cleaved caspase-9 and PARP. (B) Cells were treated with 80 μM decursin for 0, 6, 18 or 24 h and Western blotting was performed for cleaved caspase-3, cleaved caspase-9 and PARP. (C) Cells were treated with or without 80 μM decursin for 24 h and immunostained for cleaved caspase-3. The immunostained cells were mounted in medium containing DAPI and visualized under a Carl Zeiss LSM5 confocal microscope. (D) Cells were pretreated with or without caspase-3 inhibitor for 1 h and then treated with 80 μM decursin for 24 h. Western blotting was performed for cleaved caspase-3 and PARP. (E) Cells were treated with 80 μM decursin for 0, 6, 18 or 24 h and Western blotting was performed for c-IAP1, c-IAP2, Bcl-2 and Bcl-xL.
3.4. Decursin downregulates COX-2/survivin pathway in KBM-5 cells
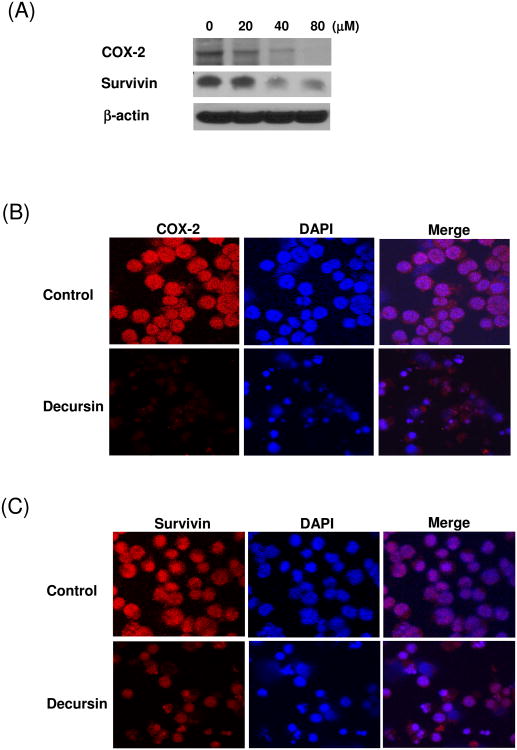
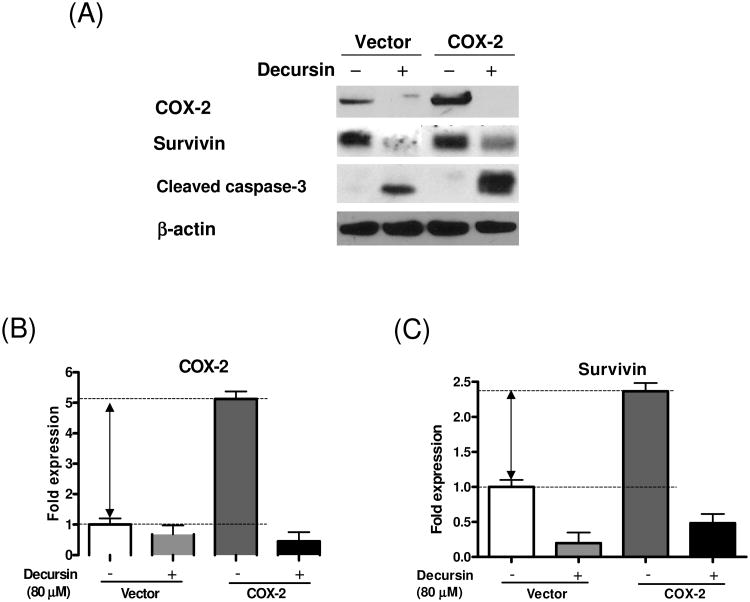
COX-2 and survivin promote the survival of tumor cells [13-15]. Studies also showed that COX-2 is closely associated with survivin [16,17]. Since decursin remarkably inhibited the expression of survivin in KBM5 cells, we tried to examine possible roles of COX-2 in decursin-induced apoptosis. Decursin treatment decreased COX-2 expression in a concentration-dependent manner (Fig. 4A). These results were further confirmed by immunofluorescence ay (Fig. 4B and C). KBM5 cells were transiently transfected with empty vector or COX-2 in the absence or presence of decursin and the effect of decursin on the expression of COX-2 and survivin was evaluated. Overexpression of COX-2 enhanced the level of survivin and the treatment with decursin inhibited the expression of both endogenous and exogenous COX-2 as well as surviving (Fig.5). These data suggested the involvement of COX-2 and survivin in decursin-induced apoptosis.
Fig. 4.
Effect of decursin on expression of COX-2 and survivin in KBM-5 cells. (A) Cells were treated with 80 μM decursin for 0, 6, 18 or 24 h and Western blotting was performed for COX-2 and survivin. (B-C) Cells were treated with or without 80 μM decursin for 24 h and immunostained for (B) COX2 and (C) survivin. The immunostained cells were mounted in medium containing DAPI and visualized under a Carl Zeiss LSM5 confocal microscope.
Fig. 5.
Effect of decursin on expression of COX-2 and survivin in wildtype and COX-2 overexpressing KBM-5 cells. (A) Cells were transiently transfected with empty vector or pSPORT6-COX2 and treated with or without decursin (40 μM) for 24 h after transfection. Cleared lysates were prepared and subjected to Western blot analysis for COX-2, survivin, cleaved caspase-3 and (β-actin. (B-C) Bar graphs represent fold differences of the COX-2 (B) and survivin (C) expression.
3.5. COX-2 inhibitors enhances decursin-induced apoptosis in KBM-5 cells
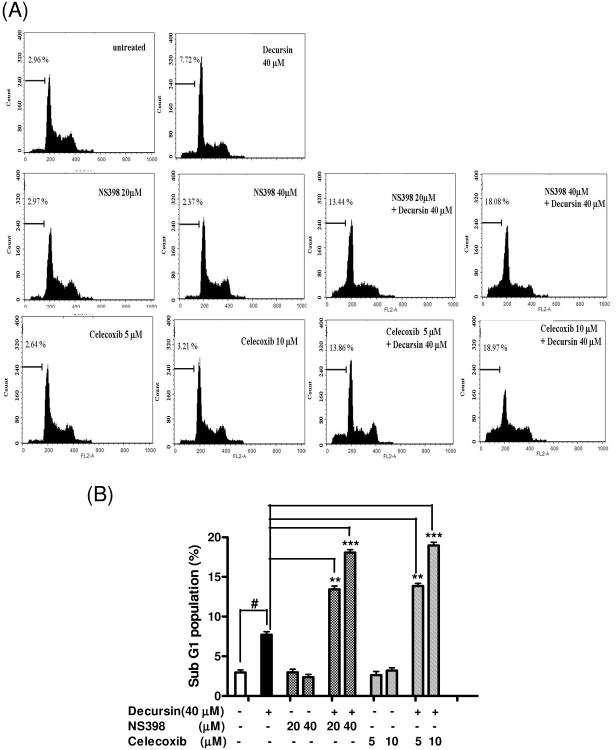
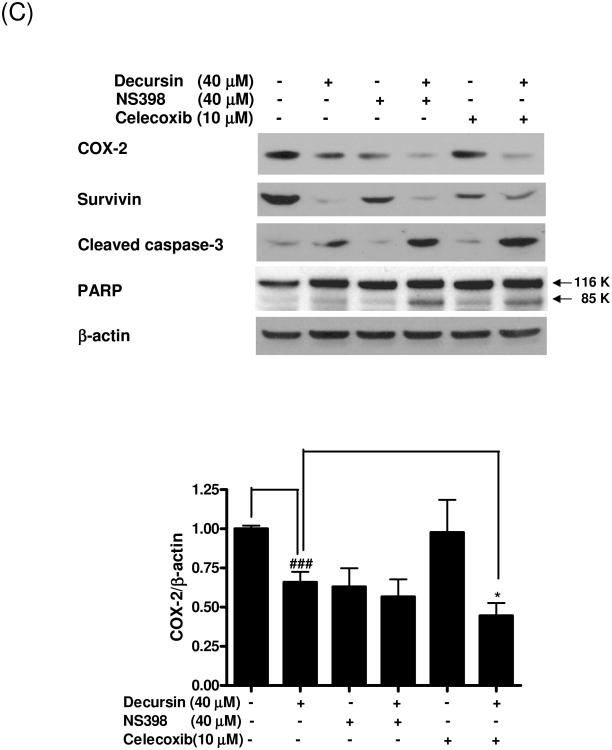
To further confirm the role of COX-2 inhibition in decursin-induced apoptosis in KBM-5 cells, we performed cell cycle analysis in KBM 5 cells treated with decursin in the absence or presence of celecoxib or NS398. As shown in Fig. 6A and B, COX-2 inhibitors, celecoxib or NS398 dramatically enhanced decursin-increased sub G1 population, while COX-2 inhibitors alone did not increase apoptosis in KBM-5 cells. Likewise, decursin promoted COX-2 inhibition, caspase-3 cleavage and PARP cleavage as well as attenuated the expression of survivin, indicating the synergistic effect of decursin and COX-2 inhibitors on the induction of apoptosis in KBM-5 cells (Fig. 6C).
Fig. 6.
Synergistic effect of decursin and COX-2 inhibitors on apoptosis in KBM-5 cells. Cells were treated with decursin (40 μM) and/or celecoxib (5 or 10 μM) or NS398 (10 or 20 μM) for 24 h. (A) For cell cycle analysis, cells were stained with PI after fixing in 75% ethanol. DNA contents of sub-G1 were analyzed by flow cytometry to determine apoptotic cell populations. (B) Bar graphs represent percentages of the sub-G1 cell population by cell cycle analysis. The data represent mean ± S.D. Statistically significant difference was calculated by Student's t-test. #, p < 0.05 versus control, **, p < 0.01 and ***, p < 0.001 versus decrusin treated cells. (C) Western blot analyses were performed using cleared cell lysates for COX-2, survivin, cleaved caspase-3 and PARP. Bar graphs represent fold expression of COX-2. The data represent mean ± S.D. Statistically significant difference was calculated by Student's t-test. ###, p < 0.001 versus control, *, p < 0.05 versus decrusin treated cells.
4. Discussion
Decursin is a compound isolated from Korean angelica (Angelica gigas) root and its antitumor effects have been reported in various cancer cells. In human prostate carcinoma cells, including DU145, PC-3, and LNCaP cells, decursin inhibited cell growth and induced cell death and cell cycle arrest at G1 phase [18]. Anti-androgen and anti-androgen receptor (AR) activities of decursin or decursin-containing herbal extracts have also been demonstrated in prostate cancer cells [10]. Recently, Jiang and colleagues demonstrated that decursin and decursinol angelate inhibited estrogen-stimulated and estrogen-independent growth, and survival of breast cancer cells [6]. However, the molecular mechanisms of decursin in CML have not been fully explored yet.
In the current study, we report that decursin induced apoptosis via downregulation of COX-2-dependent survivin pathway in KBM-5 myeloid leukemia was confirmed by the apoptotic features of cell shrinkage and blebbing, and apoptotic bodies in decursin-treated KBM-5 cells. The results indicated that decursin did not induce necrosis rather apoptosis in KBM-5 cells.
Caspase family, aspartate-specific cysteine proteases, plays a central role in regulation of apoptosis [19]. Activation of caspase-3 and following cleavage of its substrates such as PARP and lamin A is one of hallmarks of apoptosis [20]. In mitochondria-dependent apoptotic signaling, caspase-9 associates with cytochrome c and Apaf-1 to form the apoptosome (a large ternary protein structure), where caspase-9 is activated and further trigger cleavage of other caspases including caspase-3 [21]. In our study, we have found that decursin facilitated the cleavage of caspase-3, -9 and PARP in a dose-dependent manner. This suggested that intrinsic apoptotic machinery was switched on which accounts for decursin-induced cytotoxicity. Since caspase-3 inhibitor, z-DEVD-fmk, not only blocked the caspase 3 activation but also PARP cleavage induced by decursin, it suggests that caspase-3 plays a crucial role in the initiation of decursin-induced apoptosis.
Apoptosis signaling is regulated by various pro- and anti-apoptotic proteins. Bcl-2 family proteins is localized in mitochondria and control caspase activation [22]. Decursin attenuated the expression of anti-apoptotic protein Bcl-2, but not Bcl-XL in KBM-5 cells. Another family of apoptosis-regulatory proteins, inhibitors of apoptosis proteins (IAPs), are recently considered a valuable target to modulate apoptotic cell death in many cancer cells. IAP family proteins regulate apoptosis by binding and inhibiting caspases [23,24]. In our study, the expression of survivin, a member of IAP family proteins, was significantly suppressed in decursin treated KBM-5 cells. In contrast, c-IAP1 and c-IAP2 were not affected by decursin in KBM-5 cells.
Survivin has been known to be a key regulator of apoptosis [25]. Owing to unique structure of survivin, it is considered a potent target for chemo- and radiotherapy of cancer [13,26,27]. In KBM-5 cells, it was recently reported that targeting survivin could overcome the resistance against imatinib, a bcr-abl inhibitor. Survivin antisense oligonucleotide (Sur-AS-ODN) inhibited cell growth, cell cycle arrest and apoptotic cell death [28]. In addition, there are several evidences that survivin is closely associated with COX-2 in cancer cells. For instance, Krysan and colleagues reported that COX-2 dependent expression of survivin was critical for apoptosis resistance in non-small cell lung cancer [16]. Also, Erkanli suggested that COX-2 and survivin were overexpressed and positively correlated in endometrial carcinoma [29]. In our study, we observed that the level of survivin was increased when COX-2 was overexpressed in KBM-5 cells, indicating that COX-2 positively regulates survivin expression. Consistently, decursin attenuated the expression of COX-2 and survivin in parental or COX-2-transfected KBM-5 cells. Furthermore, COX-2 coordinated with survivin expression upon decursin treatment, indicating the regulatory role of COX-2 in survivin (Fig.3D)
COX-2 is an enzyme that is often overexpressed in cancer as well as in inflammation or heart diseases [30-32]. COX-2 selective inhibitors such as celecoxib (Celebrex or Celebra), valdecoxib (Bextra), NS398 and rofecoxib (Vioxx) are non-steroidal anti-inflammatory drugs (NSAID) to directly target COX-2 [33]. Recent studies reported COX-2 inhibitors can reduce carcinogenic risks in prostate, lung and breast cancers and can be utilized for the combination therapy with anticancer agents to improve the therapeutic and cost effectiveness [34]. We employed COX-2 inhibitor celecoxib or NS398 to evaluate the effect on decursin-treated KBM-5 cells. Interestingly, the combination treatment of decursin and COX-2 inhibitor celecoxib or NS398 synergistically increased the apoptotic population, the cleavages of caspase-3 and PARP as well as reduced survivin expression in KBM-5 cells. Our data suggest that decursin and COX-2 inhibitors are in synergy to promote apoptosis in KBM-5 cells.
Taken together, these findings suggested that decursin induces apoptosis via downregulation of the COX-2 dependent survivin pathway in human leukemic KBM-5 cells and also that decursin may be a potent cancer chemotherapeutic agent, the effect of which can be dramatically augmented by its combination with COX-2 inhibitors for treating human myeloid leukemia. However, although survivin was involved in decursin induced apoptosis, its protein level did not correlate well with apoptosis in the synergistic assays (Fig.6C), suggesting that other target(s) may also be involved in the synergistic apoptotic process.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2009-0063466). Dr. Chen is supported by NIHR01 CA100498, CA124490 and FAMRI-CIA.
Footnotes
Conflict of interest statements: The authors have no conflicts of interest to disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207–219. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 2.Bedi A, Zehnbauer BA, Barber JP, Sharkis SJ, Jones RJ. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- 3.K H, Chi HJ. Studies on the components of Umbelliferae plants in Korea: pharmacological study of decursin, decursinol and nodakenin. Korean J Pharmacog. 1970:25–32. [Google Scholar]
- 4.Konoshima M, Chi HJ, Hata K. Coumarins from the root of Angelica gigas Nakai. Chem Pharm Bull (Tokyo) 1968;16:1139–1140. doi: 10.1248/cpb.16.1139. [DOI] [PubMed] [Google Scholar]
- 5.Ahn KS, Sim WS, Kim IH. Decursin: a cytotoxic agent and protein kinase C activator from the root of Angelica gigas. Planta Med. 1996;62:7–9. doi: 10.1055/s-2006-957785. [DOI] [PubMed] [Google Scholar]
- 6.Jiang C, Guo J, Wang Z, Xiao B, Lee HJ, Lee EO, et al. Decursin and decursinol angelate inhibit estrogen-stimulated and estrogen-independent growth and survival of breast cancer cells. Breast Cancer Res. 2007;9:R77. doi: 10.1186/bcr1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HH, Sik Bang S, Seok Choi J, Han H, Kim IH. Involvement of PKC and ROS in the cytotoxic mechanism of anti-leukemic decursin and its derivatives and their structure-activity relationship in human K562 erythroleukemia and U937 myeloleukemia cells. Cancer Lett. 2005;223:191–201. doi: 10.1016/j.canlet.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Lee YS, Jung SH, Shin KH, Kim BK, Kang SS. Anti-tumor activities of decursinol angelate and decursin from Angelica gigas. Arch Pharm Res. 2003;26:727–730. doi: 10.1007/BF02976682. [DOI] [PubMed] [Google Scholar]
- 9.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Lee HJ, Li GX, Guo J, Malewicz B, Zhao Y, et al. Potent antiandrogen and androgen receptor activities of an Angelica gigas-containing herbal formulation: identification of decursin as a novel and active compound with implications for prevention and treatment of prostate cancer. Cancer Res. 2006;66:453–463. doi: 10.1158/0008-5472.CAN-05-1865. [DOI] [PubMed] [Google Scholar]
- 11.Kim HH, Ahn KS, Han H, Choung SY, Choi SY, Kim IH. Decursin and PDBu: two PKC activators distinctively acting in the megakaryocytic differentiation of K562 human erythroleukemia cells. Leuk Res. 2005;29:1407–1413. doi: 10.1016/j.leukres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29:489–496. doi: 10.1016/j.cellbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli M, Seveno C, Bataille R, Barille-Nion S. Survivin in cancerology: molecular aspects and therapeutic applications. Med Sci (Paris) 2008;24:821–827. doi: 10.1051/medsci/20082410821. [DOI] [PubMed] [Google Scholar]
- 14.Sakoguchi-Okada N, Takahashi-Yanaga F, Fukada K, Shiraishi F, Taba Y, Miwa Y, et al. Celecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cells. Biochem Pharmacol. 2007;73:1318–1329. doi: 10.1016/j.bcp.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Younis T, Hache KD, Rayson D, Dewar R, Gray S, Barnes PJ. Survivin and COX-2 expression in male breast carcinoma. Breast. 2009;18:228–232. doi: 10.1016/j.breast.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–6362. doi: 10.1158/0008-5472.CAN-04-1681. [DOI] [PubMed] [Google Scholar]
- 17.Maxia C, Perra MT, Demurtas P, Minerba L, Murtas D, Piras F, et al. Relationship between the expression of cyclooxygenase-2 and survivin in primary pterygium. Mol Vis. 2009;15:458–463. [PMC free article] [PubMed] [Google Scholar]
- 18.Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005;65:1035–1044. [PubMed] [Google Scholar]
- 19.S G, Stennicke HR. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- 20.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 22.Zamzami N, Brenner C, Marzo I, Susin SA, Kroemer G. Subcellular and submitochondrial mode of action of Bcl-2-like oncoproteins. Oncogene. 1998;16:2265–2282. doi: 10.1038/sj.onc.1201989. [DOI] [PubMed] [Google Scholar]
- 23.Richter BW, Duckett CS. The IAP proteins: caspase inhibitors and beyond. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.44.pe1. [DOI] [PubMed] [Google Scholar]
- 24.Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10:169–177. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- 25.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 26.Capalbo G, Rodel C, Stauber RH, Knauer SK, Bache M, Kappler M, et al. The role of survivin for radiation therapy. Prognostic and predictive factor and therapeutic target. Strahlenther Onkol. 2007;183:593–599. doi: 10.1007/s00066-007-1800-4. [DOI] [PubMed] [Google Scholar]
- 27.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–476. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 28.Carter BZ, Mak DH, Schober WD, Cabreira-Hansen M, Beran M, McQueen T, et al. Regulation of survivin expression through Bcr-Abl/MAPK cascade: targeting survivin overcomes imatinib resistance and increases imatinib sensitivity in imatinib-responsive CML cells. Blood. 2006;107:1555–1563. doi: 10.1182/blood-2004-12-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erkanli S, Bolat F, Kayaselcuk F, Demirhan B, Kuscu E. COX-2 and surviving are overexpressed and positively correlated in endometrial carcinoma. Gynecol Oncol. 2007;104:320–325. doi: 10.1016/j.ygyno.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 30.Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001;134:1137–1150. doi: 10.1038/sj.bjp.0704365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobadilla RV, Barnett EM, Randels CL. COX-2 inhibitors and the heart: putting risk in perspective. Adv Nurse Pract. 2005;13:48–50. [PubMed] [Google Scholar]
- 32.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Mol Interv. 2006;6:199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 33.Teran-Estrada L, Miranda-Limon JM, Galvan-Villegas F. Cardiovascular risk of cyclooxygenase selective inhibitors. Rev Med Inst Mex Seguro Soc. 2008;46:287–299. [PubMed] [Google Scholar]
- 34.Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20:615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]