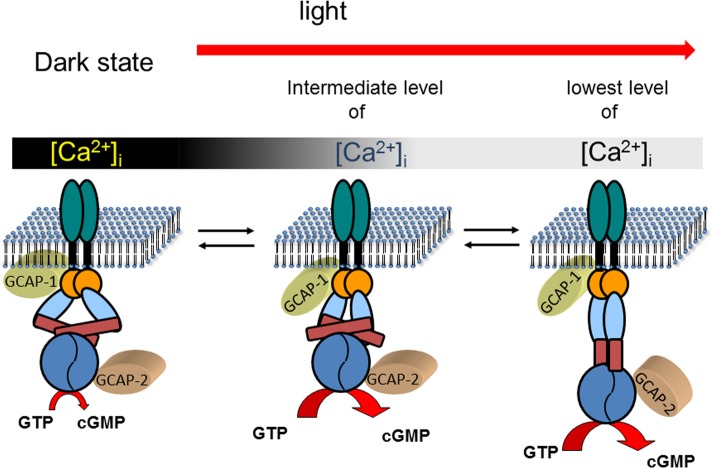
Figure 5.
Ca2+-relay model of sequential GCAP action. Photoreceptor guanylate cyclases are dimers with one transmembrane domain in each monomer. In addition, each monomer consists of one extracelllar domain (in rods, this domain is in the lumen of the disks), one juxtamembrane domain (orange), and one kinase homology domain (light blue). Further, the dimerization domain (dark red rectangle) is important for the formation of an active enzyme controlling the correct positioning of the two catalytic domains (blue). In the dark state of the cell, GCAPs are fully or partially saturated with Ca2+ which keeps the guanylate cyclase activity at a very low level that is sufficient to keep a fraction of the CNG-channels in the plasma membrane open. GCAPs form with the target guanylate cyclase a complex in the presence and absence of Ca2+, which enables a rapid response to changing Ca2+-concentration after illumination. When the intracellular Ca2+-concentration falls to an intermediate level, Ca2+ dissociates form GCAP1. This process triggers a conformational change in GCAP1, leading to the activation of guanylate cyclase. When Ca2+ reaches its final lower intracellular level, GCAP2 turns into an activator. The different conformations of the guanylate cyclase are hypothetical and need to be verified in future experiments. It is further suggested that GCAPs stabilize the transition states of the cGMP catalytic step, but experimental proof is also lacking so far. The stepwise and reversible action of GCAPs would allow the cell to react on small incremental changes in Ca2+ with a fine-tuned response system.