Figure 7.
Slit-Induced Changes in Growth Cone F-Actin and Cofilin Are PS Dependent
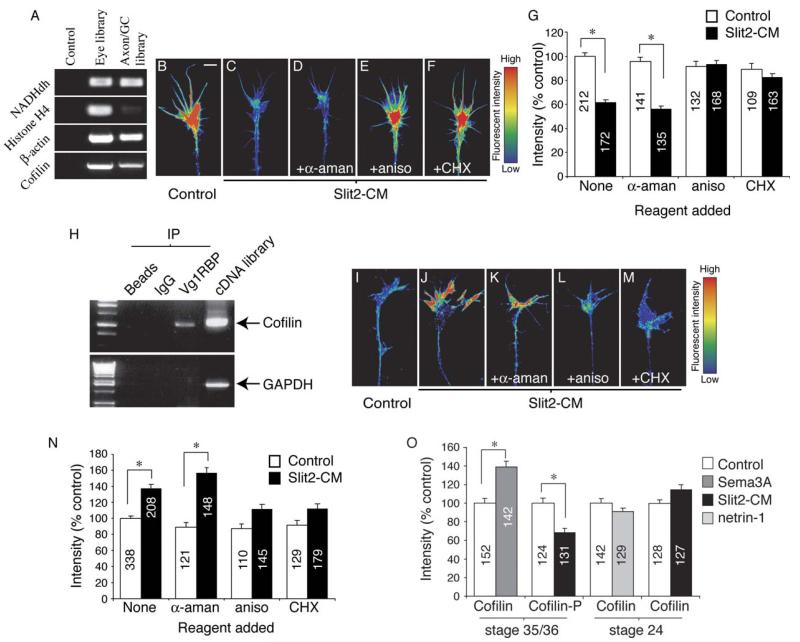
cDNA libraries were constructed from mRNA extracted from cultured stage 35/36 eyes or isolated stage 35/36 cultured axons and growth cones. β-actin and cofilin were identified from both samples by using PCR (A). Using digital quantitation of immunofluorescence, Slit2-CM was shown to cause an ~40% decrease in growth cone F-actin fluorescent intensity (B, C, and G). This decrease was dependent on translation, but not transcription (D–G). Cofilin mRNA was immunoprecipitated with Vg1RBP bound to protein-A beads. Beads alone or beads coupled to rabbit IgG did not immunoprecipitate cofilin mRNA. GAPDH mRNA did not immunoprecipitate with Vg1RBP. Xenopus cDNA was used as a PCR positive control (H). Slit2-CM significantly raised total growth cone cofilin immunoreactivity after 5 min. This increase was not seen when translation was inhibited by aniso or CHX (I-N). Sema3A also elicited a rise in growth cone cofilin immunoreactivity, while phosphorylated cofilin (cofilin-P) immunofluorescence decreased in response to Slit2-CM. Cultured stage 24 growth cones stimulated with netrin-1 or Slit2-CM did not demonstrate a significant change in total cofilin fluorescent intensity (O). Numbers inside bars denote growth cones tested. Growth cone images have been pseudocolored to represent fluorescent intensity: low intensity, blue; high intensity, red. *p < 0.001, Kruskal-Wallis test. Scale bar: 5 μm.