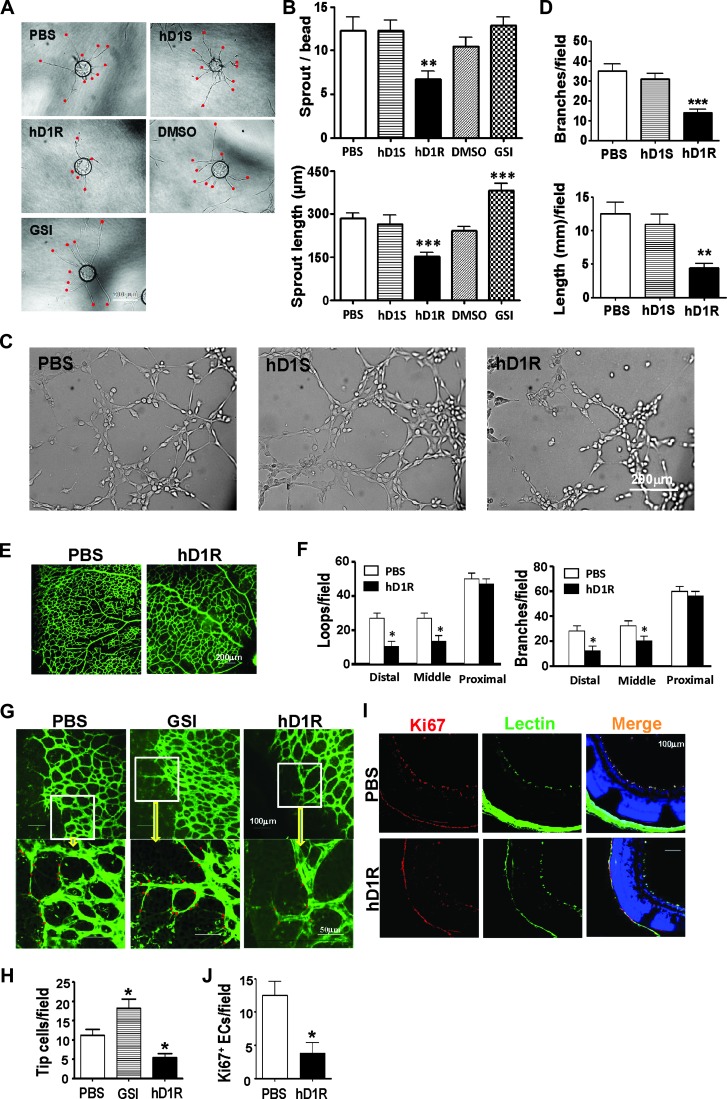
Figure 2.
hD1R repressed angiogenesis both in vitro and in vivo. (A, B) HUVECs weremixed with Cytodex 3 microbeads (400 cells per bead) and cultured for 24 hours for angiogenic sprouting in the presence of PBS, hD1R, hD1S, DMSO, or GSI. Each sprout was labeled with a red dot (A) and the numbers of sprouts and the sprout length per bead were counted and compared (B). (C, D) HUVECs were cultured in 48-well dishes coated with Matrigel Basement Membrane Matrix in the presence of PBS, hD1S, or hD1R. Cells were cultured under the indicated conditions for 3 hours and photographed under a microscope (C). The formation of endothelial networks was quantified by counting branch points and length of cells along the lumens (D) and compared. (E, F) P3 pups were injected daily s.c. with PBS or hD1R. On P7, the retinas of the pups were collected, flat-mounted, and stained with fluorescein-labeled Griffonia simplicifolia Lectin I. The structures of the whole retinal vasculature were shown (E). The numbers of enclosed capillary loops and vessel branch points were compared (F). (G, H) P3 pups were injected daily s.c. with PBS, GSI, or hD1R. On P7, the retinas of the pups were collected, flat-mounted, and stained with fluorescein-labeled Griffonia simplicifolia Lectin I. Tip cells at the angiogenic fronts were counted (G) and compared (H). (I, J) P3 pups were injected daily s.c. with PBS or hD1R. On P7, the retinas of the pups were collected and sectioned. The samples were co-stained with anti-Ki67 (red) and lectin (green) and were counterstained with Hoechst (I). The Ki67+ signals were compared (J). Bars, means ± SD. *P < .05, **P < .01, ***P < .001, n = 5.