Figure 6.
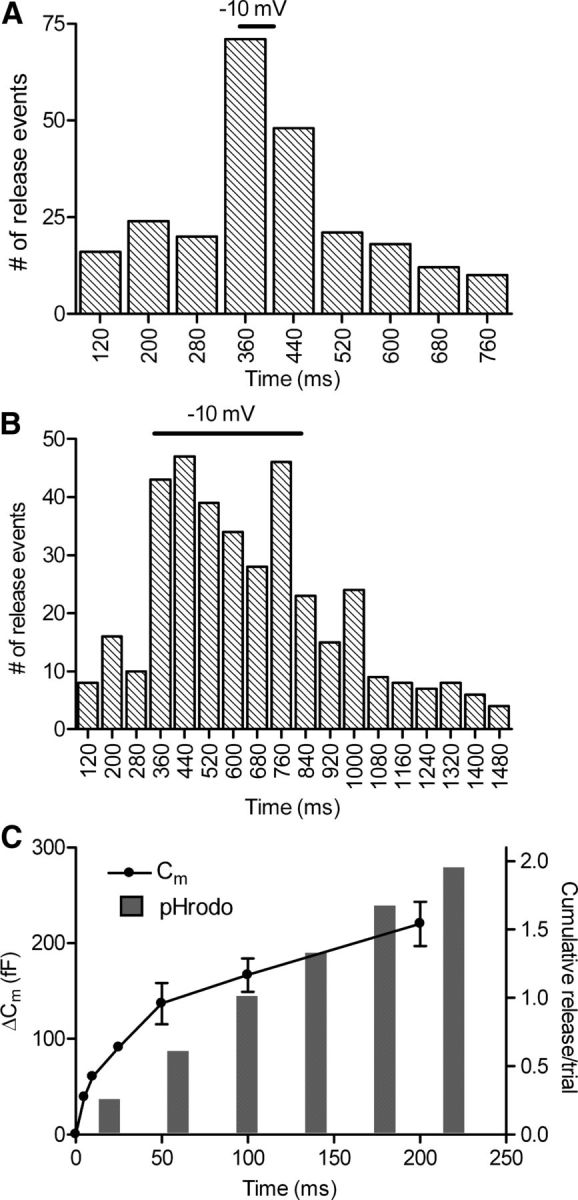
Kinetics of exocytosis measured optically was similar to that measured by electrophysiological techniques. Rods loaded with dextran-conjugated pHrodo were depolarized from −70 to −10 mV for 50 ms (A) or 500 ms (B). The timing of the TTL trigger pulse sent from the imaging computer jittered between two frames, occurring during images acquired either 330 or 370 ms into the trial. To accommodate this frame-to-frame jitter, the number of release events was binned in 80 ms increments. The rate of release rose and declined rapidly during the 50 ms test step (A; n = 70 trials in 32 rods). Release also increased rapidly with the 500 ms test step and declined after the end of the step (B; n = 55 trials in 20 rods). C, A comparison of the cumulative increase in fusion events measured optically with cumulative fusion measured electrophysiologically from changes in rod membrane capacitance. Membrane capacitance increases were evoked by depolarizing test steps to −10 mV for durations of 5, 10, 25, 50, 100, and 200 ms applied to rods in a retinal slice preparation (n = 7, circles). The amplitude of the capacitance jump was measured over a 5 ms window beginning 30 ms after termination of the test step. For optical measurements of release, the cumulative histogram of release events evoked by 500 ms test steps to −10 mV (data in B) was binned at 40 ms intervals (bars).
