Figure 1.
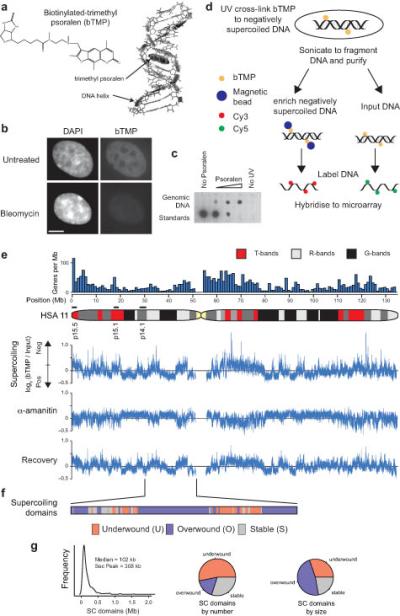
High resolution mapping of DNA supercoiling. (a) Cartoon showing biotinylated-trimethyl psoralen (bTMP)51 as a DNA structure probe that intercalates into under-wound regions of the DNA helix1, 39. (b) Immunofluorescence analysis in RPE1 cells showing the distribution UV cross-linked bTMP detected using NeutraAvidin-Fluorescein before and after bleomycin treatment. Cells were counterstained with DAPI (Bar is 5 μm). (c) Dotblot showing bTMP incorporated into genomic DNA detected by streptavidin conjugated horseradish peroxidase and chemiluminescence. (d) Experimental strategy for high resolution mapping of DNA supercoiling. Cells were treated with bTMP for 20 mins and the bTMP was cross-linked into the DNA helix with 360 nm UV light. DNA was purified, enriched for biotin-TMP using streptavidin coated magnetic beads, amplified, labeled and hybridized to genomic microarrays versus input control. (e) Microarray data showing bTMP binding across human (HSA) chromosome 11 as log2(bTMP binding/Input DNA) revealing pronounced differences in drug binding representative of differences in DNA supercoiling. Transcription inhibition with α-amanitin (5 hours) showed substantial remodeling of DNA supercoiling that was reversed upon drug washout (2 hours recovery). (f) Diagram showing supercoiling domains across 20 Mb of HSA Chr11p identified as regions that were remodeled after transcription inhibition and categorized as “under-wound”, “over-wound” or “stable”. (g) Size distribution of supercoiling domains across regions studied and pie charts showing size and frequency distribution of different domain categories.
