Abstract
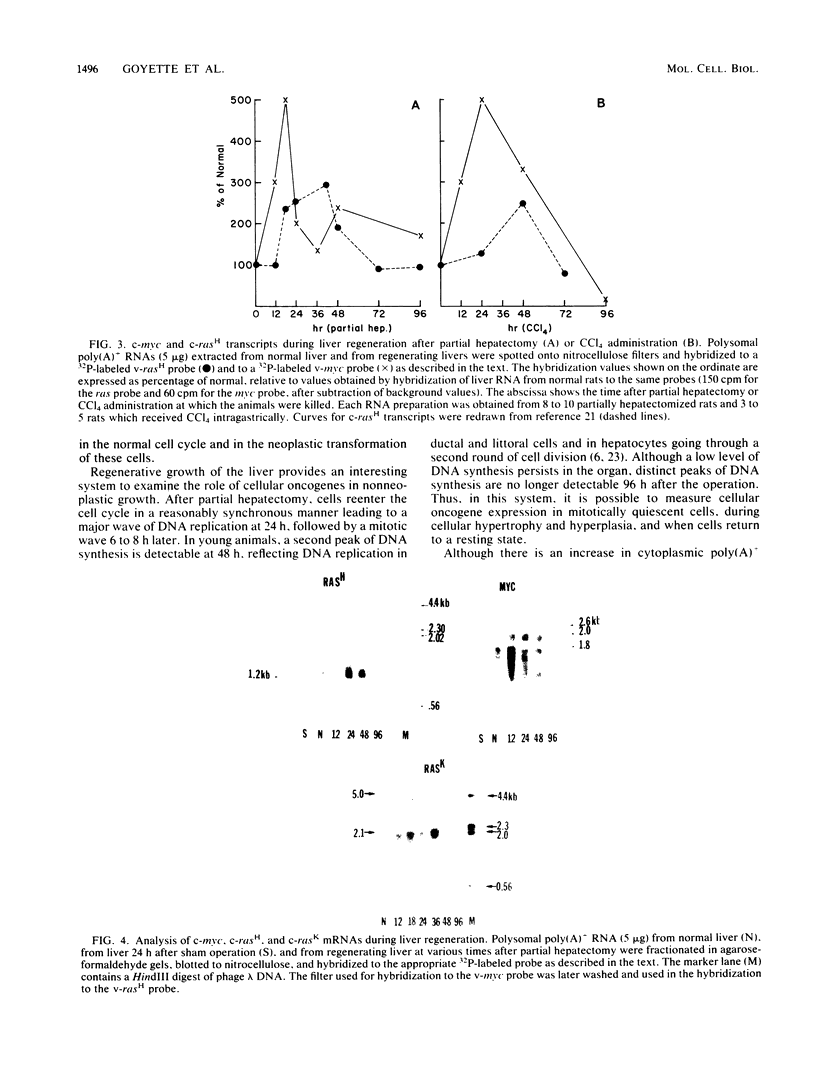
We examined the transcription of six cellular oncogenes during the process of compensatory growth in rat liver after partial hepatectomy. We have previously reported that transcripts of c-rasH are elevated during regenerative growth of the liver. We now report that transcripts of c-rasK and c-myc genes are significantly elevated after partial hepatectomy, whereas transcripts of c-abl and c-src are essentially unchanged and transcripts of c-mos are undetectable in either normal or regenerating rat liver. In liver regeneration after partial hepatectomy or chemical injury, changes in c-myc transcripts occur before DNA synthesis. The elevation of c-myc and c-ras transcripts is sequential in that highest levels of c-myc transcripts were detected 12 to 18 h after partial hepatectomy, whereas the levels of c-rasH and c-rasK were maximal by 36 to 48 h. Transcripts of all three activated oncogenes returned to their basal levels by 96 h.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atryzek V., Fausto N. Accumulation of polyadenylated mRNA during liver regeneration. Biochemistry. 1979 Apr 3;18(7):1281–1287. doi: 10.1021/bi00574a025. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Lemire J. M., Halvorson H. O. Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):839–853. doi: 10.1128/mcb.3.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Krontiris T. G., Cooper G. M. Transforming genes of human bladder and lung carcinoma cell lines are homologous to the ras genes of Harvey and Kirsten sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3637–3640. doi: 10.1073/pnas.79.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Cooper G. M., Ritz J., Lane M. A. Identification and molecular cloning of the human Blym transforming gene activated in Burkitt's lymphomas. Nature. 1983 Sep 8;305(5930):112–116. doi: 10.1038/305112a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Furth M. E., Scolnick E. M. Mouse cells contain two distinct ras gene mRNA species that can be translated into a p21 onc protein. Mol Cell Biol. 1982 Nov;2(11):1339–1345. doi: 10.1128/mcb.2.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Eva A., Aaronson S. A. Frequent activation of c-kis as a transforming gene in fibrosarcomas induced by methylcholanthrene. Science. 1983 May 27;220(4600):955–956. doi: 10.1126/science.6302839. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fausto N. Messenger RNA in regenerating liver: implications for the understanding of regulated growth. Mol Cell Biochem. 1984;59(1-2):131–147. doi: 10.1007/BF00231309. [DOI] [PubMed] [Google Scholar]
- Fausto N., Schultz-Ellison G., Atryzek V., Goyette M. Distribution and specificity of sequences in polyadenylated nuclear RNA of normal, regenerating, and neoplastic liver. J Biol Chem. 1982 Mar 10;257(5):2200–2206. [PubMed] [Google Scholar]
- Fausto N., Shank P. R. Oncogene expression in liver regeneration and hepatocarcinogenesis. Hepatology. 1983 Nov-Dec;3(6):1016–1023. doi: 10.1002/hep.1840030621. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubin G., Goldman D. S., Luce J., Neiman P. E., Cooper G. M. Molecular cloning and nucleotide sequence of a transforming gene detected by transfection of chicken B-cell lymphoma DNA. Nature. 1983 Mar 10;302(5904):114–119. doi: 10.1038/302114a0. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Grady L. J., Campbell W. P., North A. B. Sequence diversity of nuclear and polysomal polyadenylated and non-polyadenylated RNA in normal and regenerating rat liver. Eur J Biochem. 1981 Apr;115(2):241–245. doi: 10.1111/j.1432-1033.1981.tb05229.x. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Maguire R. T., Robins T. S., Thorgeirsson S. S., Heilman C. A. Expression of cellular myc and mos genes in undifferentiated B cell lymphomas of Burkitt and non-Burkitt types. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1947–1950. doi: 10.1073/pnas.80.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. S., Toole J. J., Cunningham J. M., Chang E. H., Lowy D. R., Weinberg R. A. Characterization of a human colon/lung carcinoma oncogene. Nature. 1983 Mar 3;302(5903):79–81. doi: 10.1038/302079a0. [DOI] [PubMed] [Google Scholar]
- Mushinski J. F., Bauer S. R., Potter M., Reddy E. P. Increased expression of myc-related oncogene mRNA characterizes most BALB/c plasmacytomas induced by pristane or Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1073–1077. doi: 10.1073/pnas.80.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Verma I. M., Adamson E. D. Expression of c-onc genes: c-fos transcripts accumulate to high levels during development of mouse placenta, yolk sac and amnion. EMBO J. 1983;2(5):679–684. doi: 10.1002/j.1460-2075.1983.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. F., Overell R. W. Fibroblast immortality is a prerequisite for transformation by EJ c-Ha-ras oncogene. Nature. 1983 Aug 18;304(5927):648–651. doi: 10.1038/304648a0. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Weinberg R. A. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Mol Cell Biol. 1983 Dec;3(12):2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C., Andrews G., Tamaoki T., Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983 Apr 25;258(8):4901–4906. [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Aaronson S. A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982 Dec 9;300(5892):539–542. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Scholla C. A., Tedeschi M. V., Fausto N. Gene expression and the diversity of polysomal messenger RNA sequences in regenerating liver. J Biol Chem. 1980 Apr 10;255(7):2855–2860. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes P. R., Birnie G. D., Paul J. Changes in nuclear and polysomal polyadenylated RNA sequences during rat-liver regeneration. Nucleic Acids Res. 1979;6(6):2193–2208. doi: 10.1093/nar/6.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]