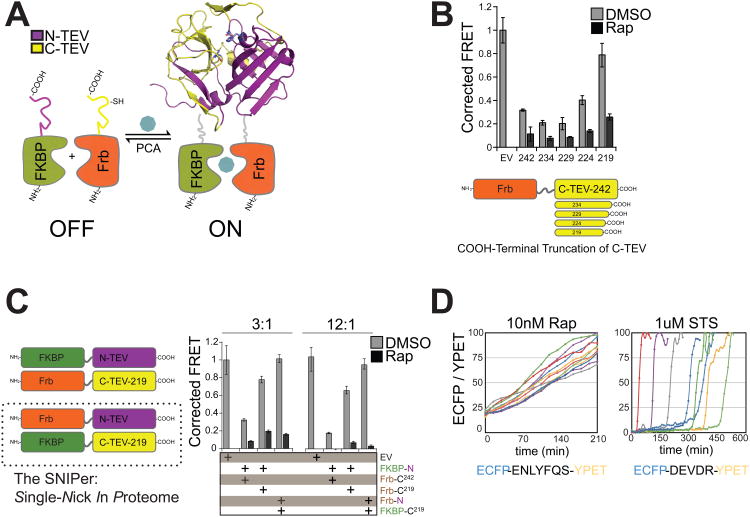
Figure 1. Engineering the SNIPer for Conditional Proteolysis.
(A) Generalized scheme for a ligand-inducible orthogonal protease based on the protein complementation system. (B) Plasmids with deletions at the C-terminus of Frb-C-TEV or empty vector (EV) were co-transfected with FKBP-N-TEV and ECFP-TevS-YPET in 293T cells. 10nM Rap was added for 12h and FRET measurements were recorded using a fluorescent microplate reader. Data are presented as corrected averages of FRET/ECFP from an experiment performed in quadruplicate (Error Bars±SD). (C) N-TEV and C-TEV-219 were fused to either FKBP or Frb and assayed by FRET in quadruplicate as above. Transfections were performed with 3× or 12× molar excess of Tev constructs to reporter plasmid (Error Bars±SD). (D) The kinetics and cell-to-cell heterogeneity of SNIPer-mediated protease activity were compared to endogenous caspase activation by live-cell fluorescent imaging. HEK293 cells were transfected with the SNIPer and ECFP-TevS-YPET or ECFP-DEVDR-YPET, a reporter for caspase-3/-7 activity in cells, and treated with 10nM Rap or 1uM staurosporine. Live-cell FRET measurements were recorded every 15 minutes. See also Figure S1.