Fig. 1.
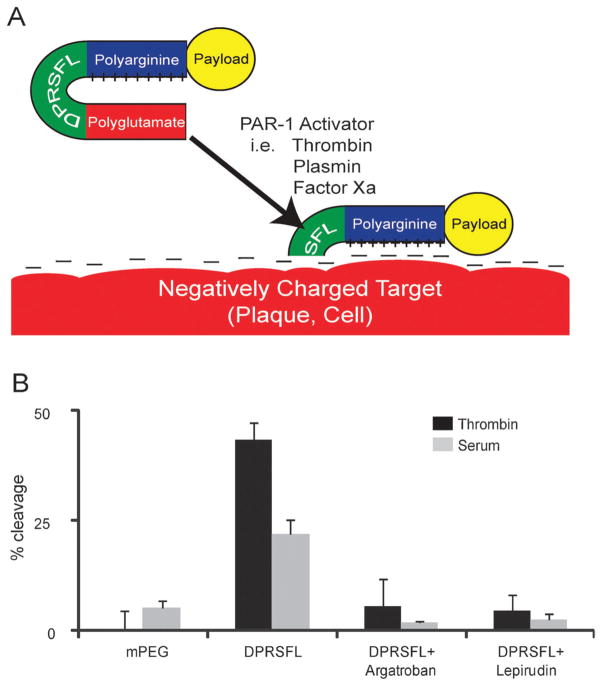
Protease cleavage of DPRSFL–ACPP is thrombin dependent and promotes uptake of fluorescent payload into cells and plaques. Schematic of an ACPP showing a positively charged polyarginine separated from a negatively charged polyglutamate by DPRSFL, a thrombin cleavable linker derived from the PAR-1 receptor (A). Once the linker is cleaved, the polyarginine is free to associate with negatively charged targets on cellular membranes, within atherosclerotic plaques or other tissue. Analysis of proteolytic cleavage of the DPRSFL–ACPP after 20 minutes incubation at 37 °C with either 50 nM thrombin (dark grey) or 50% mouse serum (light grey) in the presence and absence of the direct thrombin inhibitors argatroban and lepirudin (B) (see Fig. S1A (ESI†) for gel analysis). DPRSFL–ACPP was proteolytically cleaved by both thrombin and serum but not plasma. In each case cleavage was >90% inhibited by either argatroban or lepirudin. Also included are data showing that the negative control mPEG–ACPP is not cleaved by either thrombin or serum. Quantitation of data was done as shown in Fig. S1B (ESI†) and all experiments were done in triplicate.