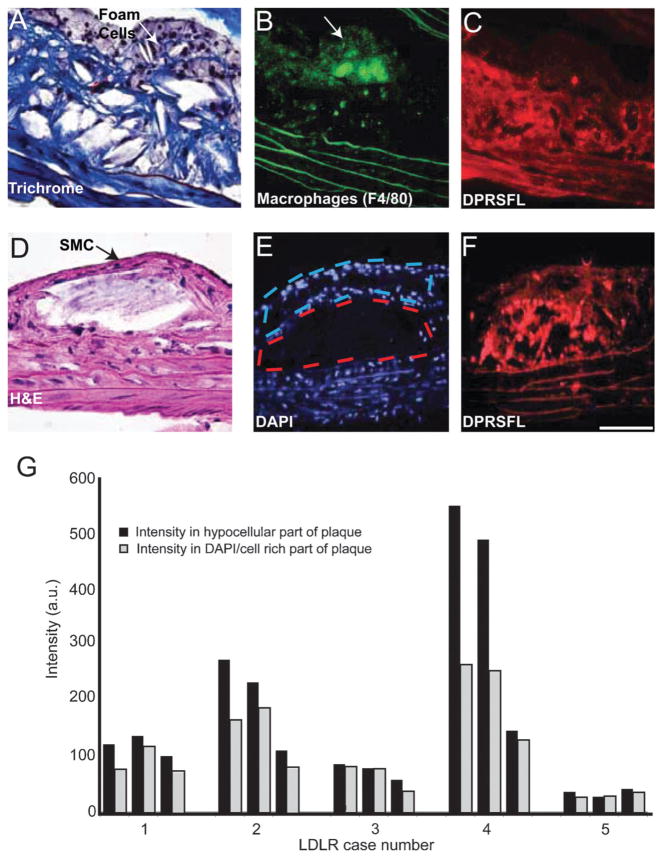
Fig. 4.
Thrombin activity, detected by DPRSFL–ACPP labeling, is localized in the deeper fibro-collagenous region of plaque and away from the foam cells. (A–F) Adjacent sections (A–C) from one plaque and (D–F) from another plaque from LDLR−/− mice. Trichrome (A) and H–E (D) staining show an interior core of fibrous tissue and a superficial layer of foam/smooth muscle cells. Macrophage identity was confirmed by F4/80 staining and coincided with the superficial foam cell layer (B). Cy5 uptake resulting from cleavage of DPRSFL–ACPP is localized primarily to the deeper fibrous layer (C,F). The histological sections (A,D) highlight the finding that although these two representative plaques are distinct in their morphology, they both show a similar localization of DPRSFL–ACPP fluorescence within the interior/deeper neointima of the plaques. Sections were stained with DAPI to differentiate the nucleus-rich foam cells/smooth muscle cell layer with nucleus-poor interior fibrous layers (E). This information was used to quantify the differential Cy5–DPRSFL–ACPP distribution (n = 5 mice, three sections collected at 100 micrometre intervals per mouse, G). p = 0.02, Student’s t-test, 2 tailed paired (scale bar: A,D = 60 micrometres, C,D and E,F = 100 micrometres).