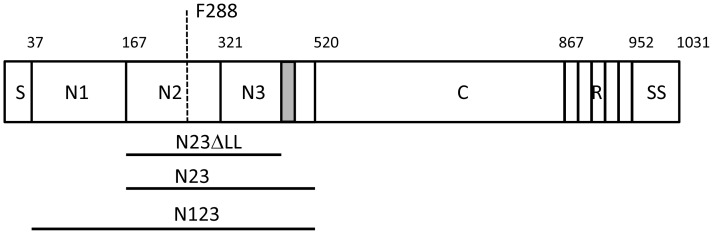
Figure 1. Schematic diagram of SpsD.
The A domain modelled on the structure of ClfA spans residues 37–519 following the secretory signal sequence S. This is followed by a connecting region C (residues 521–867) and a repeat region R in strain ED99. The number of repeats varies from strain to strain resulting in proteins of slightly different sizes. A sorting signal (SS: LPXTG motif, hydrophobic domain and positively charged residues) occurs at the extreme C-terminus. The minimum ligand binding domain construct N2N3 spans residues 167–519. Residues comprising the ‘lock’ and ‘latch’ (grey box residues 490–499) are indicated (N23ΔLL). Residue F288 that is predicted to lie within the ligand binding trench is indicated by the dashed line.