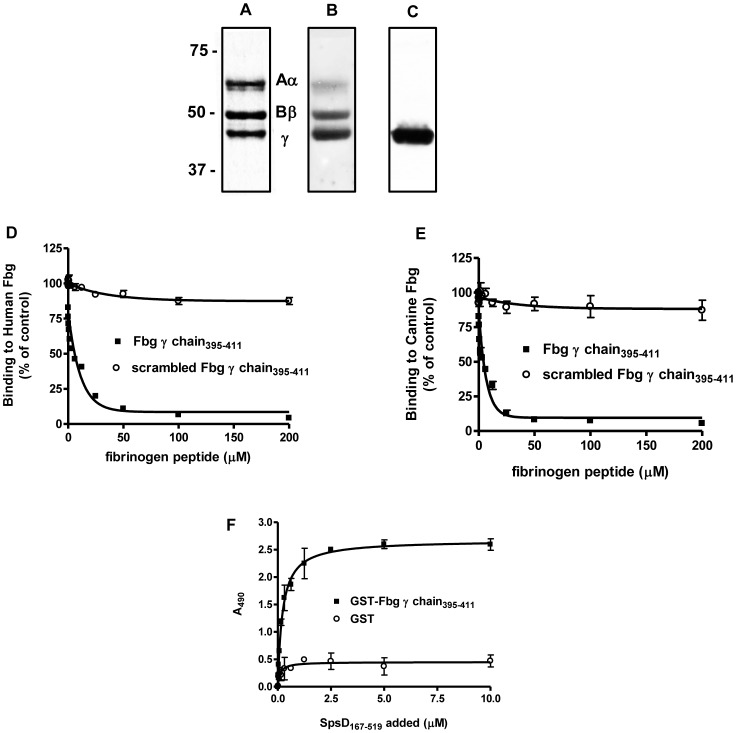
Figure 4. SpsD recognizes Fbg γ chain.
(A), human Fbg was subjected to SDS-PAGE in reducing conditions and then stained with Coomassie Blue. (B), Fbg was subjected to SDS-PAGE in reducing conditions, transferred to a nitrocellulose membrane and Fbg chains detected by incubation of the membrane with anti-mouse Fbg IgG and then HRP-conjugated rabbit anti-mouse IgG. (C), Fbg was subjected to SDS-PAGE under reducing conditions, electroblotted onto nitrocellulose filter and probed with SpsD167–519 followed by incubation with mouse anti-SpsD IgG and HRP-conjugated rabbit anti-mouse IgG. (D) and (E), effect of the Fbg C-terminal γ chain peptide on the binding of SpsD167–519 protein to immobilized Fbg. Surface-coated human (D) or canine (E) Fbg was incubated with SpsD167–519 in the presence of increasing concentrations of the 17- mer synthetic peptide of the γ chain. The effect of a scrambled peptide on Fbg-SpsD167–519 interaction is also reported as control; the assay was performed as in (C). (F), dose-dependent binding of SpsD167–519 protein to immobilized GST fused to the Fbg C-terminus of γ chain. Microtiter wells were coated with GST-Fbg γ chain and probed with increasing concentrations of SpsD167–519. Complex formation was detected by addition of mouse anti- 6-His IgG followed by HRP-conjugated rabbit anti-mouse IgG. Values are the means ± S.E of triplicate wells and are representative of one experiment. This experiment was performed three times with similar results.