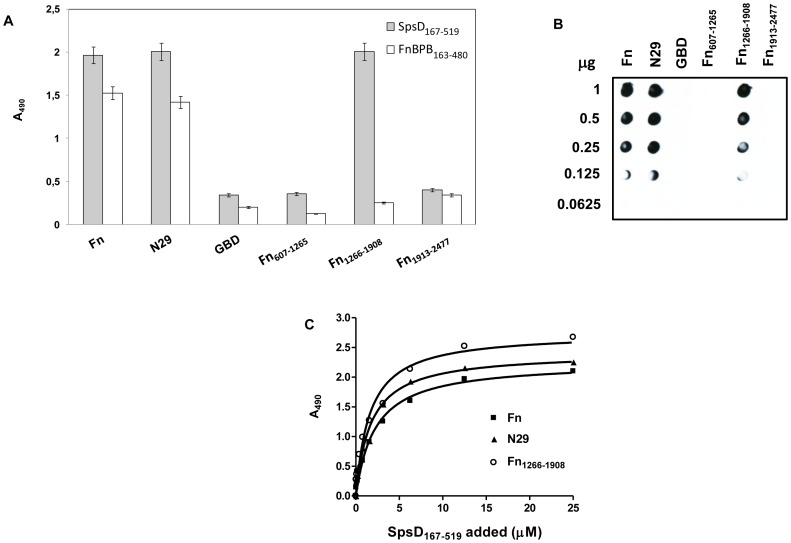
Figure 6. Localization of SpsD binding sites in Fn.
(A), native Fn, the N29, the GBD, the recombinant fragments Fn607–1263, Fn1266–1908 and the C-terminal region Fn1913–2477 of Fn were immobilized onto a microtiter plate and then incubated with of SpsD167–519. Bound protein was detected by addition to the wells of anti-SpsD37–519 mouse antibody, followed by HRP-conjugated rabbit anti-mouse antibody. Binding of FnBPB163–480 to Fn and its fragments reported as control was detected by addition to the wells of anti-FnBPB163–480 rabbit IgG, followed by HRP-conjugated goat anti-rabbit antibody. (B), different amounts of Fn and Fn fragments (1000-62.5 ng) were dotted onto a nitrocellulose membrane, probed with SpsD167–519 (1 µg/ml) and complexes detected by addition of mouse anti-SpsD37–519 IgG, followed by HRP-conjugated rabbit anti-mouse IgG. (C), surface-coated Fn, the N29 fragment and the region including the aa residues 1266–1908 were incubated with increasing concentrations of SpsD167–519 and complex formation detected as reported in (A). Results shown in panels A and C are the mean values of triplicate samples ± S.E. The experiments were repeated three times with similar results.