Abstract
Strategies to encapsulate cells in cytocompatible 3-dimensional hydrogels with tunable mechanical properties and degradability without harmful gelling conditions are highly desired for regenerative medicine applications. Here we reported a method for preparing poly(ethylene glycol)-co-polycarbonate hydrogels through copper-free, strain-promoted azide-alkyne cycloaddition (SPAAC) “Click” chemistry. Hydrogels with varying mechanical properties were formed by “clicking” azido-functionalized poly(ethylene glycol)-co-polycarbonate macromers with dibenzocyclooctyne functionalized poly(ethylene glycol) under physiological conditions within minutes. Bone marrow stromal cells encapsulated in these gels exhibited higher cellular viability than those encapsulated in photo-crosslinked poly(ethylene glycol) dimethacrylate. The precise control over the macromer compositions, the cytocompatible SPAAC crosslinking, and the degradability of the polycarbonate segments combined make these hydrogels promising candidates for scaffold- and stem cell-assisted tissue repair and regeneration.
Keywords: hydrogel, ring-opening polymerization, strain-promoted azide-alkyne cycloaddition, cell encapsulation, rheology
Introduction
Biocompatible hydrogels play an important role in biomedical research and pharmaceutical applications.1-8 They have been used as protein microchips,9 drug and gene delivery carriers,10 ophthalmic prostheses,11 and scaffolds for encapsulating cells to facilitate either the investigation of cell-extracellular matrix interactions or tissue regenerations.12-21 Naturally occurring biopolymers such as collagens, fibrin, alginate, agarose, hyaluronan and chondroitin sulfate, as well as synthetic polymers such as poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), poly(N-isopropylacrylamine) (PNIPAAM) have been used for regenerative medicine applications.12
It has been recognized that the chemistry, microstructure and physical properties of hydrogel tissue scaffolds have significant influences on the fate of their resident cells.22-24 From this perspective, synthetic hydrogels present unique advantages over naturally occurring hydrogels due to the broader tunability of the properties of the former.25-27 Challenges still exist, however, for the translation of existing synthetic hydrogels for biomedical uses. For instance, the gelling of most physically crosslinked hydrogels requires substantial changes in environmental conditions (e.g., pH, temperature, ionic strength), which could be detrimental to the in situ encapsulated cells. In addition, the integrity of these physically crosslinked cell-gel constructs could be difficult to maintain in vivo. On the other hand, the cytotoxicity of crosslinking reagents and initiators used for chemically crosslinked hydrogels can negatively impact the viability and long-term fate of the encapsulated cells.28-30 In general, chemical crosslinking conditions and chemically crosslinked networks deemed cyto-compatible are still limited.31 Among them, PEG-based hydrogels formed by photo-initiated radical polymerization of (meth)acrylated PEG macromers have been the most utilized for the encapsulation and support of tissue-specific differentiation of stem cells. Major limitations associated with photo-crosslinked PEG gels include the intrinsic heterogeneities of the network structures due to the uncontrolled radical polymerization process and the varied degrees of cytotoxicity of the aqueous radical initiators utilized (e.g., I-2959 and VA-086).29 Alternative in situ crosslinking strategies involving disulfide bond formations or Michael addition reactions between thiols and acrylates or vinyl sulfones can eliminate the need for radical initiators, but still suffer from the potential interference from the thiol residues widely present within the tissue environment. Thus, a hydrogel system that can be crosslinked under physiological conditions without external perturbations or cross-reactivities with cellular or tissue environment is highly desired. Finally, for tissue regeneration applications, these hydrogels should also ideally possess adequate mechanical properties and exhibit tunable degradation rates potentially matching with those of the tissue integrations.
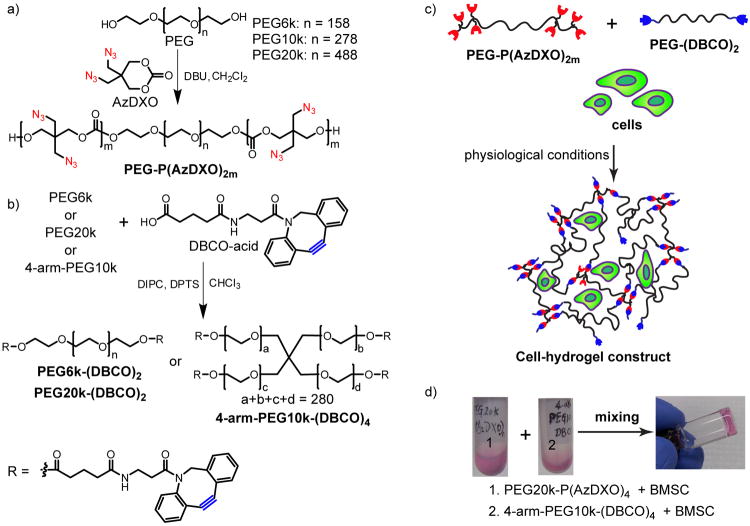
Here, we report a facile method for preparing PEG macromers flanked with aliphatic azido-functionalized biodegradable polycarbonate blocks (Fig 1a), which are subsequently crosslinked with dibenzocyclooctyne (DBCO)-terminated PEG macromers (Fig 1b) using copper-free, strain-promoted [3+2] azide-alkyne cycloaddition (SPAAC) (Fig 1c). The choice of the SPAAC “click” chemistry 32, 33 as the in situ crosslinking strategy is inspired by the high fidelity and orthogonality of the reaction as well as its compatibility with physiological conditions. Equally important, comparing to the copper-catalyzed [3+2] azide-alkyne cycloaddition (CuAAC) that have been more broadly applied to the functionalization of hydrogels including PEG,34 PVA 35 and hyaluronan,36 the copper-free SPAAC presents significant advantage in terms of both short-term cytocompatibility and long-term biocompatibility. Although more biocompatible metal catalysts are being developed for CuAAC,37 their safety for in vivo tissue engineering applications remains unknown. By contrast, the SPAAC has already been utilized for live cell imaging,38 in vivo metabolic labelling in C. elegans,39 zebrafish40, 41 and mice.42 Thus, it is not surprising that SPAAC has quickly caught the attention of the polymer/hydrogel and tissue engineering communities.43, 44
Figure 1.
Macromer synthesis, crosslinking and cell encapsulation strategies. a) Ring-opening polymerization (ROP) of AzDXO initiated by PEG with the catalyst of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). [AzDXO] = 0.1125 M, [DBU] = 0.1 M, rt, Ar, 4 h; b) Synthesis of PEG-(DBCO)x by reacting DBCO-acid with PEG in CH2Cl2 under the catalysis of DIPC and DPTS, rt, 10 h; c) Depiction of the cell encapsulation by crosslinking PEG-P(AzDXO)2m and PEG-(DBCO)x via SPAAC “click” reaction; d) A representative demonstration of the rapid gellation of the cell-hydrogel constructs within 1 min of mixing the BMSC cell suspension (106 cells/mL) in a PEG20k-P(AzDXO)4 solution (10 w/v% in expansion media) and a 4-arm-PEG10k-DBCO solution (10 w/v% in expansion). The BMSC expansion media consisted of α-MEM without ascorbic acid and 20% FBS.
The azido-functionalized polycarbonate blocks, which serve as SPAAC crosslinking sites, are grafted to the ends of the hydrophilic PEG using organocatalytic ring-opening polymerization (ROP) of an easy-to-prepare functional cyclic carbonate monomer, AzDXO, that we recently developed.45 ROP of cyclic monomers has been widely used for preparing biodegradable polymers. Recent progress on the development of organocatalysts for ROP 46 offers great potential for preparing hydrogels with reduced toxicity that are associated with traditional metal catalysts.47, 48 The previously demonstrated (co)polymerization versatility of AzDXO by living organocatalytic ROP under mild conditions (e.g., rt) and the facile functionalization of the side chains of the resulting polycarbonate P(AzDXO) via SPAAC 45 under physiological conditions have opened the possibilities for adjusting the mechanical, biochemical and degradation properties of the PEG-co-P(AzDXO) hydrogels for regenerative medicine applications. This study presents the preparation, mechanical property characterization and cytocompatibility of the hydrogels crosslinked by SPAAC.
Results and Discussion
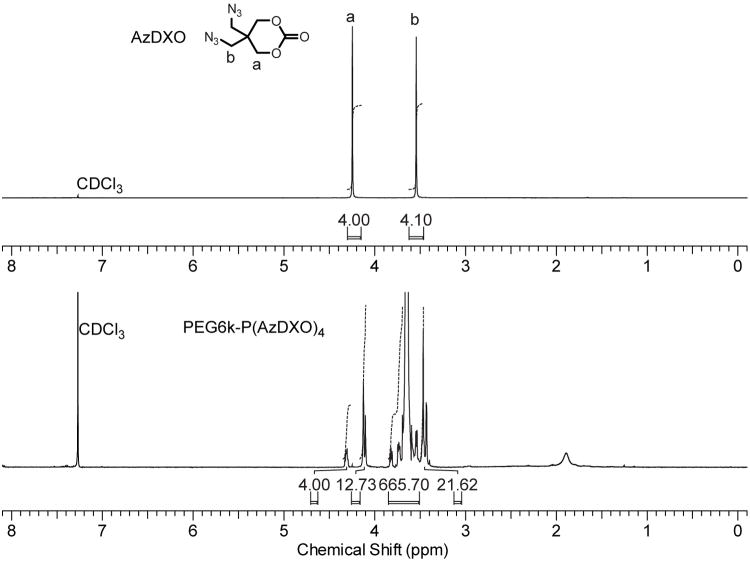
The AzDXO monomer was synthesized in 2-steps as previously described.45 Living/controlled ROP of AzDXO (0.1125 M) was initiated by varying amount of PEG6k, PEG10k and PEG20k using organocatalyst 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in dichloromethane (0.01 M) at rt (Fig. 1a). The conversion of monomer reached ∼90% in 4 h. Upon termination by benzoic acid, the PEG-P(AzDXO)2m macromer products were purified by repeated precipitation from dichloromethane in ethyl ether with >95% overall yield. As shown in Table 1, the P(AzDXO) block lengths (or degree of polymerization, DP) in the resulting tri-block macromers PEG-P(AzDXO)2m as determined by 1H NMR (Fig. 2) were close to the theoretical values (2m) calculated assuming 100% monomer conversion. GPC results revealed very narrow polydispersity of all PEG-P(AzDXO)2m (PDI: 1.02-1.09) with various PEG and P(AzDXO) block length combinations. The water solubility of macromer PEG-P(AzDXO)2m was dependent on the overall length of the P(AzDXO) blocks (Table 1). When the overall P(AzDXO) block length was shorter than 8 repeating units, the resulting PEG-P(AzDXO)2m was soluble in water regardless of the length of the PEG block. By contrast, the triblock macromers became insoluble in water when the degree of polymerization of AzDXO was above the critical number of 8 regardless of the length of the initiating PEG (MW 6,000, 10,000 or 20,000). Similar phenomenon was observed in other amphiphilic triblock copolymers initiated by PEG. For example, Hiemstra et.al found that the triblock PEG-(PLA)2 copolymers were insoluble in water when the lactic acid repeating units were greater than 22, regardless of the PEG block length investigated.49
Table 1. Characterizations of PEG-P(AzDXO)2m macromers.
| Name a | DPb | MnNMRc | Mn/N3d | MnGPCe | PDI e | Water solubility |
|---|---|---|---|---|---|---|
| PEG6k-P(AzDXO)4 | 4.2 | 7909 | 946 | 12357 | 1.02 | soluble |
| PEG6k-P(AzDXO)7 | 6.8 | 8465 | 622 | 12641 | 1.03 | soluble |
| PEG6k-P(AzDXO)8 | 8.4 | 8805 | 523 | 12931 | 1.03 | cloudy |
| PEG6k-P(AzDXO)11 | 10.8 | 9313 | 431 | 13168 | 1.03 | insoluble |
| PEG10k-P(AzDXO)11 | 11.1 | 14658 | 662 | 20956 | 1.03 | insoluble |
| PEG20k-P(AzDXO)4 | 3.6 | 22338 | 3079 | 36563 | 1.08 | soluble |
| PEG20k-P(AzDXO)9 | 9.4 | 23573 | 1248 | 33380 | 1.09 | swellable |
The naming of the samples reflects the approximate copolymer compositions including the averaged molecular weight of the initiating PEG and the degree of polymerization of the polycarbonate blocks;
degree of polymerization of AzDXO determined from 1H NMR;
number-averaged molecular weight calculated from 1H NMR;
number-averaged molecular weight per azido group;
number-averaged molecular weight and polydispersity index determined by GPC using an evaporative light scattering (ELS) detector.
Figure 2.
Representative 1H NMR spectra and proton integrations of PEG6k-P(AzDXO)4 (bottom) supporting successful polymerization of AzDXO (top) initiated from the end hydroxyl groups of PEG6k. Proton integrations support a copolymer composition approximating that of the theoretical estimate.
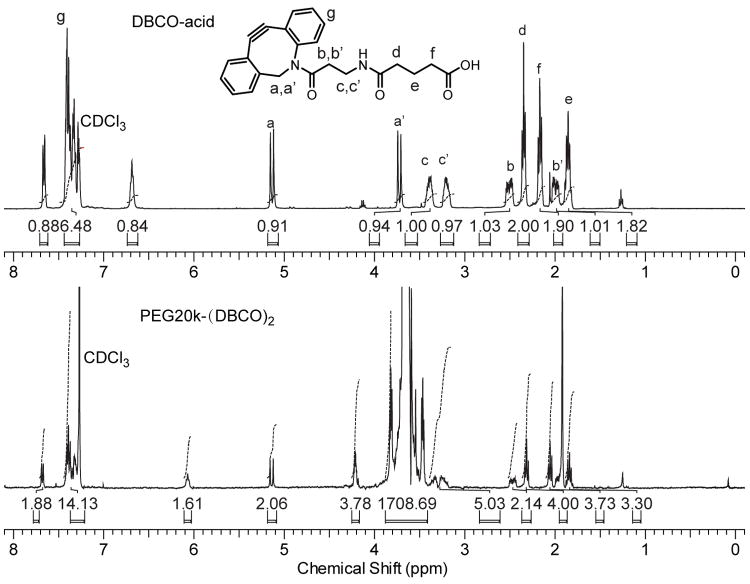
The linear or 4-arm DBCO-terminated PEG macromers, PEG-(DBCO)x (x = 2 or 4), were synthesized by end-capping PEGs of various molecular weights and architectures (PEG6k, PEG20k or 4-arm-PEG10k) with DBCO-acid via esterification. The reaction were carried out in anhydrous dichloromethane with catalysts N,N′-diisopropycarbodimide (DIPC) and 4-(dimethylamino)-pyridinium p-toluenesulfonate (DPTS). The reactant ratio was kept as 1:1.5:5:0.25 / hydroxyl end-groups in PEG : DBCO-acid : DIPC : DPTS. Complete esterification of the PEG end-groups was supported by the disappearance of the characteristic 13C NMR signal for -CH2-OH from 61.4 ppm in the 13C NMR spectra of PEG-(DBCO)x (Supporting Information, Fig. S1). The catalysts and by-products were readily removed by washing with water and subsequently precipitating in diethyl ether, or via sequential dialysis precipitation against diethyl ether and water. An overall high yields of >90% were obtained. As representatively shown in Figure 3, the 1H NMR peak at 2.17 ppm corresponding to the methylene protons of -CH2COOH of DBCO-acid was shifted to 2.05 ppm upon esterification with PEG20k, and a new peak at 4.22 ppm corresponding to the methylene protons of -CH2OCO- in the resulting PEG20k-(DBCO)2 appeared. The peak at 6.07 ppm in PEG20k-(DBCO)2 and the peak at 6.67 ppm in DBCO-acid corresponded to the respective amide protons, the chemical shifts and intensities of which varied significantly with their concentrations and the water content in the NMR solvent. The 13C NMR (Supporting Information, Fig. S1) along with the 1H NMR integrations (Fig. 3) supported the successful attachment of DBCO-acid to all hydroxyl termini of the PEG.
Figure 3.
Representative 1H NMR spectra and proton integrations of PEG20k-(DBCO)2 (bottom) supporting the successful attachment of DBCO-acid (top) on both ends of PEG20k.
Water soluble PEG-P(AzDXO)2m and PEG-(DBCO)x were dissolved in cell culture media, and in the presence of cell suspension, were readily mixed to form elastic cell-hydrogel construct under physiological conditions (Fig 1d). Six different hydrogels were prepared using two PEG-P(AzDXO)2m macromers and three PEG-(DBCO)x macromers with different structures, compositions, and molecular weights for this study.
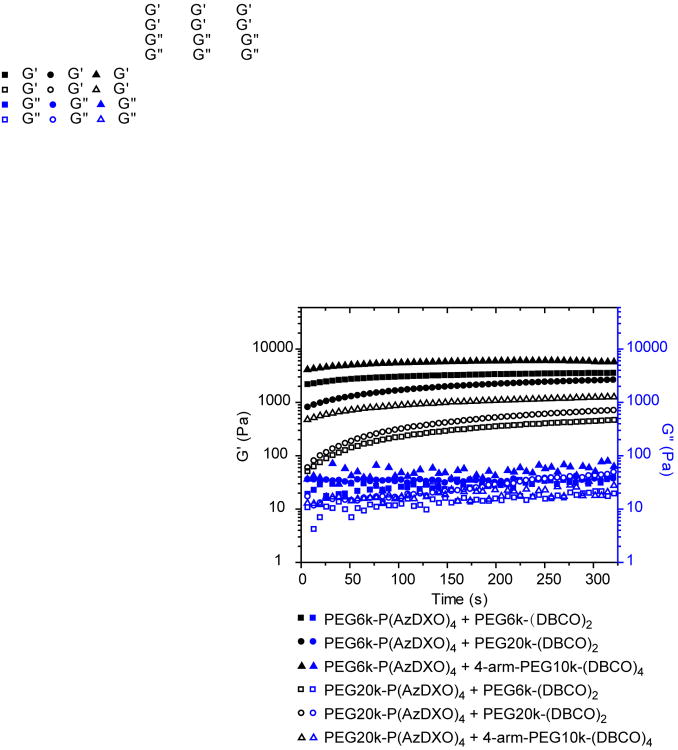
To study the SPAAC crosslinking process between the PEG-P(AzDXO)2m macromers and the PEG-(DBCO)x macromers and to determine the shear moduli of the crosslinked gels, time-sweep oscillatory rheology tests were carried out on the 6 formulations of the respective macromers (Fig. 4). Briefly, PEG-(DBCO)2 or 4-arm-PEG-(DBCO)4 solution (10 w/v%) was first loaded on the bottom plate of 20-mm parallel plates equipped with a Peltier heating unit (AR-2000 Rheometer, TA Instruments), before the aqueous solution of PEG-P(AzDXO)2m (10 w/v%) was added and rapidly mixed on plate by a pipette. The mixtures were equilibrated at 37 °C between the plates for 1 min prior to the test to ensure consistency among various formulations. As shown in Figure 4, both the storage moduli (G′) and loss moduli (G″) of the mixtures increased with time and the recorded values levelled off after 300 sec, suggesting that the SPAAC crosslinking was completed within a matter of minutes. The sol-gel transition point, defined as the point where G′ increased to across with G″, was not observed using this testing protocol, likely due to the rapid occurrence of SPAAC crosslinking within the first minute of mixing the respective macromers. Indeed, rigorous quantitative comparisons of the gelling rates among the various fast-gelling formulations would be challenging without significant modification of the mixing and testing protocols. Qualitative observation of the gelling process by tilting the vials upon mixing the respective macromers revealed that the gelling rate followed the following trend: 4-arm-PEG10k-(DBCO)4 > PEG6k-(DBCO)2 > PEG20k-(DBCO)2 upon mixing with PEG-P(AzDXO)2m. All formulations started to gel in less than 1 min by the vial tilting test (Fig. 1d), consistent with the implications derived from the time-sweep oscillatory rheology tests (Fig. 4). Such a fast gelling rate will be beneficial for applications of the macromers as injectable hydrogel formulations to repair tissue defects where the containment of the hydrogel within the local environment is critical.
Figure 4.
Time-dependent shear storage moduli (G′, black symbols) and shear loss moduli (G″, blue symbols) of the various hydrogel formulations during the SPAAC crosslinking.
The equilibrium shear modulus of the hydrogel can be tuned by adjusting the length between the reactive groups (PEG block length) or macromer structures (linear vs. 4-arm). As shown in Figure 4, at the same weight content (10 w/v%), hydrogels crosslinked from PEG-(DBCO)x and PEG6k-P(AzDXO)4 (solid symbols) exhibited higher storage moduli than those crosslinked from PEG-(DBCO)x and PEG20k-P(AzDXO)4 (open symbols), suggesting that the storage modulus inversely correlated with the PEG length between the P(AzDXO) blocks. Among all 6 formulations, the hydrogel crosslinked by PEG6k-P(AzDXO)4 and 4-arm-PEG10k-(DBCO)4 exhibited the highest storage modulus throughout the gelling process, with its equilibrium G′ approaching 6.0 KPa. This is largely due to the highest chemical crosslinking density accomplished by the 4-armed DBCO crosslinker and the PEG-P(AzDXO)2m with the shortest PEG block length. In addition to chemical crosslinking density, degree of physical entanglement also played an important role in the storage modulus of the gel, especially when both macromers contain sufficiently long PEG blocks. For instance, the gel crosslinked by PEG20k-P(AzDXO)4 and PEG20k-(DBCO)2 exhibited higher modulus than that crosslinked by PEG20k-P(AzDXO)4 and PEG6k-(DBCO)2. Overall, the equilibrium shear moduli of the 6 gel systems decrease in the following order: PEG6k-P(AzDXO)4 + 4-arm-PEG10k-(DBCO)4 (6 KPa) > PEG6k-P(AzDXO)4 + PEG6k-(DBCO)2 (3.5kPa) > PEG6k-P(AzDXO)4 + PEG20k-(DBCO)2 (2.7 kPa) > PEG20k-P(AzDXO)4 + 4-arm-PEG10k-(DBCO)4 (1.3 kPa) > PEG20k-P(AzDXO)4 + PEG20k-(DBCO)4 (0.7 kPa) > PEG20k-P(AzDXO)4 + PEG6k-(DBCO)2 (0.5 kPa).
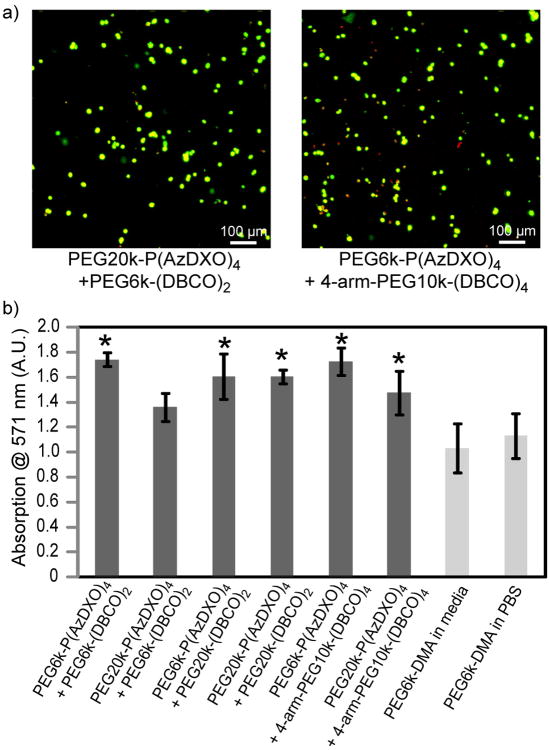
The cytocompatibility of PEG-P(AzDXO)2m and PEG-(DBCO)x macromers and the respective “click” hydrogels were evaluated in vitro. Bone marrow-derived stromal cells (BMSC) cultured in the presence of 10 w/v% of each macromer showed comparable cell viability at 48 h to those cultured without any macromer supplements (Fig. S2), supporting excellent cytocompatibility of these macromers. Further, we showed that most BMSCs encapsulated by “clicking” the macromer components (Fig. 1d) remained viable, as supported by the dominant green fluorescent stains for live cells observed at 24 h upon performing a live/dead cell staining on the cell-hydrogel constructs (Fig. 5a). No statistically significant difference in the hydrogel storage modulus was detected upon the encapsulation of BMSC in any of the formulations investigated.
Figure 5.
Viability of BMSC cells encapsulated by the “click” hydrogels. (a) Representative confocal Z-stack (400 μm) images of encapsulated BMSC cells stained with a live/dead viability staining kit 24 h after initial encapsulation. Live cells were stained green while dead cells were stained red; (b) MTT cell viability assay performed on the hydrogel-BMSC constructs (106 cells/mL) 48 h after cell encapsulation showing better cell viability for all “click” hydrogel-encapsulated BMSCs than those encapsulated in photo-crosslinked PEG6k-DMA hydrogels. * indicates p < 0.05 (student t-test) between the “click” gel and the PEG6k-DMA control (crosslinked in the media).
MTT cell viability assay performed on the hydrogel-cell constructs 48 h after cell encapsulation (Fig. 5b) showed that BMSC cells encapsulated in all “click” hydrogels (106 cells/mL) exhibited higher viability than those photo-encapsulated in the PEG6k-DMA hydrogel that was widely used for cell encapsulation in cartilage engineering.50, 51 In our hands, the gellation of the 10 w/v% PEG6k-DMA gels in the presence of BMSC (106 cells/mL) and 0.05 w/v% Irgacure-2959 photoinitiator required irradiation at 365 nm for 10 min. It is known that environmental conditions such as oxygen level could lead to variation in the required polymerization time. The consistently more rapid gelling (within 1 min for most formulations) enabled by the SPAAC crosslinking presented here, coupled with the eliminated need for toxic photoinitiators or UV irradiation, presents a significant advantage.
Conclusion
Cytocompatible PEG-co-polycarbonate hydrogels crosslinked by water soluble PEG-P(AzDXO)2m macromers with varying PEG block lengths and linear or 4-armed DBCO-capped PEG macromers were prepared using copper-free SPAAC. The macromer components were non-cytotoxic, and the rapid gelling (as quick as < 60 sec) enabled by the copper-free “click” chemistry allowed the encapsulation of bone-marrow derived stromal cells with higher cellular viability than the photo-crosslinked PEG6k-DMA gels commonly used for cartilage tissue engineering applications. The mechanical properties of these gels could be readily tuned by the adjusting macromer structures and the lengths of their constituent polymer blocks. The combination of cytocompatibility and tunable gelling rates and mechanical properties make these “clickable” gels appealing candidates as cell encapsulation strategies and as injectable formulations for minimally invasive tissue repair.
To fully explore the potential of these cytocompatible click gels for regenerative medicine applications, the degradation kinetics of the aliphatic polycarbonate blocks as a function of the gel composition is being investigated. In addition, multipotency of the encapsulated bone marrow stromal cells is also being analyzed to determine the suitability of these cell-hydrogel constructs for promoting musculoskeletal tissue regeneration.
Experimental Section
Chemicals
Azido-functionalized cyclic carbonate monomer (AzDXO) was synthesized as described previously.45 Poly(ethylene glycol) diol (PEG, Mn= 6,000, 10,000, 20,000 g/mol, Aldrich) and 4-arm-PEG (Mn = 10,000 g/mol, JenKem Technology) were dried under vacuum in melt state for 3 h prior to use. Aza-dibenzocyclooctyne acid (DBCO-acid) was purchased from Click Chemistry Tools (Macon, GA, USA). 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was purified by distillation with calcium hydride under reduced pressure. Dichloromethane and chloroform were dried by distillation over P2O5 immediately prior to use. All other chemicals were used as received.
Synthesis of PEG-P(AzDXO)2m
PEG-P(AzDXO)2m macromers were prepared by initiating ROP of AzDXO with PEG6k, PEG10k or PEG20k under the catalysis of DBU at rt in CH2Cl2. The AzDXO concentration and DBU concentration were kept at 0.1125 M and 0.01 M, respectively. The amount of PEG was adjusted accordingly to obtain various copolymer compositions shown in Table 1. In a representative procedure for synthesizing PEG6k-P(AzDXO)4, PEG6k (6.00 g, 1.00 mmol) and AzDXO (0.955 g, 4.50 mmol) were dissolved in 38 mL of CH2Cl2 under argon atmosphere. A 2-mL solution of DBU (0.2 M in CH2Cl2) was injected to initiate the polymerization. After 4 h, benzoic acid (0.122g, 1.0 mmol) was added to neutralize DBU. The polymer was purified by precipitation in 800 mL of ethyl ether. The precipitate was then redissolved in 40 mL of CH2Cl2 and precipitated again in 800 mL of ethyl ether, and repeated twice. The macromer was obtained as white powder and dried under vacuum at rt (6.740g, yield = 97%).
Synthesis of PEG-(DBCO)x
The alkyne-containing macromers PEG-(DBCO)x were synthesized by esterification of the hydroxyl ends of the respective linear PEG or 4-arm-PEG with DBCO-acid. In a representative synthesis of PEG-(DBCO)x with high Mn, PEG20k (4.0 g, ∼0.2 mmol) was dissolved in 20 mL of chloroform. DBCO-acid (234.3 mg, 0.6 mmol), 4-(dimethylamino)-pyridinium p-toluenesulfonate (DPTS, 29.4 mg, 0.1 mmol) and N,N-diisopropylcarbodiimide (DIPC, 252.4 mg, 2.0 mmol) were added subsequently. After 10 h, 80 mL of chloroform was added and the solution was washed by 20 mL of 0.1 M NaCl aqueous solution three times. The organic layer was dried with anhydrous sodium sulfate overnight. After filtering, the clear solution was dropped into 900 mL of ethyl ether, and the white precipitate was collected by filtration. The solid was redissolved in 100 mL of chloroform and reprecipitated in 900 mL of ethyl ether and repeated twice until no residue catalysts could be detected by 1H NMR. The macromer was obtained as white powder and dried under vacuum at rt (4.05g, yield = 97.4%).
NMR and GPC
1H (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Varian INOVA-400 spectrometer in deuterated chloroform (CDCl3, 99.8 atom% D with 0.03% v/v TMS). GPC measurements were taken on a Varian ProStar HPLC system equipped with two 5-mm PLGel MiniMIX-D columns (Polymer Laboratory, Amherst, MA), a UV-vis detector and a PL-ELS2100 evaporative light scattering detector (Polymer Laboratory, Amherst, MA). THF was used as an eluent at a flow rate of 0.3 mL/min at rt. The number-averaged molecular weight (Mn) and the polydispersity index (PDI) were calculated by a Cirrus AIA GPC Software using narrowly dispersed polystyrenes (ReadyCal kits, PSS Polymer Standards Service Inc. Germany) as calibration standards.
Rheology
Dynamic rheology test was performed on an AR-2000 rheometer (TA Instruments) equipped with 20-mm parallel plates and a Peltier heating unit. The gelling process of the various formulations and the evolution of the shear modulus of the hydrogels were studied by oscillatory time sweep rheology experiments at 37 °C. Aqueous solutions of PEG-P(AzDXO)2m and PEG-(DBCO)x (10 w/v%) in cell expansion media (α-MEM without ascorbic acid, 20% FBS) with 1:1 molar ratio of the azide groups to the alkyne groups were loaded on the bottom plate sequentially and mixed by pipette. The mixed solution was kept between the parallel plates for 60 sec before the experiment and data collection were initiated to ensure consistency among various formulations. An oscillatory frequency of 1 Hz and a strain of 0.5% were applied.
Bone marrow stromal cell (BMSC) encapsulations
PEG-P(AzDXO)2m and PEG-(DBCO)x were dissolved in BMSC expansion media to reach a 10 w/v% concentration, respectively. The solutions were sterile-filtered through a 0.22-μm filter. BMSC were harvested from the femur and tibia of skeletally mature male rats (Charles River SASCO SD) and enriched by adherent culture as previously described.52 Passage 1 BMSC cells were plated overnight in expansion media, trypsinized, counted and suspended into the respective macromer solutions (106 cells/mL). The two BMSC-macromer solutions, PEG-P(AzDXO)2m and PEG-(DBCO)x, were mixed in a total volume of 50 μL in 96-well tissue culture plate. An extra 200 μL of expansion media was added to each well after 45 min. As a control hydrogel for BMSC encapsulation, poly(ethylene glycol) dimethylacrylate (PEGDMA Mn ∼ 6000 g/mol) was also photo-crosslinked in the presence of BMSC cells. PEGDMA (10 w/v%) and the photo initiator Irgacure-2959 (0.05 w/v%) were dissolved in PBS (pH 7.4) or BMSC expansion media. The passage 1 BMSC cells were suspended in 50 μL of PEGDMA/Irgacure-2959 solution (106 cells/mL) and irradiated with 365 nm UV light for 10 min. An extra 200 μL of expansion media was added to each well immediately after the photo-polymerization. All cell-hydrogel constructs were cultured for 24 and 48h in a humidified incubation (5% CO2, 37 °C) before being subjected to live/dead cell staining or MTT cell viability assay. A sample size of 3 was applied to all cell-hydrogel constructs cultured for MTT.
Live and dead cell staining of the hydrogel-BMSC constructs
The hydrogel-cell constructs were stained using a LIVE/DEAD® viability/cytotoxicity kit (Molecular Probes) according the vendor's protocol. Living cells will be stained with green fluorescence by intracellular esterase catalyzed hydrolysis of Calcein AM, and dead cells will be stained red by Ethidium homodimer-1 after penetrating through the damaged membranes and binding of with nucleic acids. The stained hydrogel-cell construct was mounted on microscope slide and imaged by a Leica SP laser scanning confocal microscope. Confocal Z-stack images of encapsulated BMSC cells over the depth of 400 μm (20 consecutive 20-μm slices) were overlaid.
MTT cell viability assay
The viability of the MBSC cells cultured on tissue culture plate in the presence of 10 w/v% macromers or those encapsulated in 3-D hydrogels were evaluated using MTT cell proliferation kit (Roche) after 48-h culture in expansion media (α-MEM without ascorbic acid, 20% FBS) in 96-well plates. To a total volume of 150 μl of culture media and cell-hydrogel construct, 15 μl of MTT labelling reagent was added and incubated for 8 h at 37 °C on an orbital shaker. A 150-μl solubilization solution was then added to each well, and incubated at 37 °C on the orbital shaker for 36 h to fully dissolve and release the purple formazan crystals from the 3-D hydrogels. The absorbance at 571 nm was read on a MULTISCANFC spectrophotometer (Thermo Scientific). A sample size of 3 was applied to each construct or culture condition.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01AR055615 and R21AR056866).
References
- 1.Jen AC, Wake MC, Mikos AG. Biotechnol Bioeng. 1996;50:357. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Wang CM, Varshney RR, Wang DA. Adv Drug Delivery Rev. 2010;62:699. doi: 10.1016/j.addr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA. Tissue Eng Part B-Rev. 2010;16:577. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 4.Hynd MR, Turner JN, Shain J Biomater Sci Polym Ed. 2007;18:1223. doi: 10.1163/156856207782177909. [DOI] [PubMed] [Google Scholar]
- 5.Ifkovits JL, Burdick JA. Tissue Eng. 2007;13:2369. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 6.Khetan S, Burdick JA. Soft Matter. 2011;7:830. [Google Scholar]
- 7.Kim MS, Park SJ, Chun HJ, Kim CH. Tissue Engineering and Regenerative Medicine. 2011;8:117. [Google Scholar]
- 8.Lee KY, Mooney DJ. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 9.Bertone P, Snyder M. FEBS J. 2005;272:5400. doi: 10.1111/j.1742-4658.2005.04970.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoare TR, Kohane DS. Polymer. 2008;49:1993. [Google Scholar]
- 11.Alvarez-Lorenzo C, Yanez F, Concheiro A. J Drug Deliv Sci Tec. 2010;20:237. [Google Scholar]
- 12.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 13.Tessmar JK, Gopferich AM. Adv Drug Delivery Rev. 2007;59:274. doi: 10.1016/j.addr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Burdick JA, Prestwich GD. Adv Mater. 2011;23:H41. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minh KN, Lee DS. Macromolecular Bioscience. 2010;10:563. doi: 10.1002/mabi.200900402. [DOI] [PubMed] [Google Scholar]
- 16.Marklein RA, Burdick JA. Adv Mater. 2010;22:175. doi: 10.1002/adma.200901055. [DOI] [PubMed] [Google Scholar]
- 17.Anderson SB, Lin CC, Kuntzler DV, Anseth KS. Biomaterials. 2011;32:3564. doi: 10.1016/j.biomaterials.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Nat Mater. 2008;7:816. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Proc Natl Acad Sci U S A. 2007;104:7791. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung C, Burdick JA. Tissue Engineering Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annabi N, Nichol JW, Zhong X, Ji CD, Koshy S, Khademhosseini A, Dehghani F. Tissue Eng Part B-Rev. 2010;16:371. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutolf MP, Gilbert PM, Blau HM. Nature. 2009;462:433. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Even-Ram S, Artym V, Yamada KM. Cell. 2006;126:645. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Cushing MC, Anseth KS. Science. 2007;316:1133. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 25.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Lee JS, Chansakul T, Yu C, Elisseeff JH, Yu SM. Biomaterials. 2006;27:5268. doi: 10.1016/j.biomaterials.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, Shoichet MS. Nature Materials. 2004;3:249. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 28.Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 29.Rouillard AD, Berglund CM, Lee JY, Polacheck WJ, Tsui Y, Bonassar LJ, Kirby BJ. Tissue Engineering Part C-Methods. 2011;17:173. doi: 10.1089/ten.TEC.2009.0582. [DOI] [PubMed] [Google Scholar]
- 30.Shu XZ, Liu YC, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 31.Hennink WE, van Nostrum CF. Adv Drug Delivery Rev. 2002;54:13. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 32.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2005;127:11196. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 33.Baskin JM, Bertozzi CR. Aldrichimica Acta. 2010;43:15. [Google Scholar]
- 34.Malkoch M, Vestberg R, Gupta N, Mespouille L, Dubois P, Mason AF, Hedrick JL, Liao Q, Frank CW, Kingsbury K, Hawker CJ. Chem Commun. 2006:2774. doi: 10.1039/b603438a. [DOI] [PubMed] [Google Scholar]
- 35.Ossipov DA, Hilborn J. Macromolecules. 2006;39:1709. [Google Scholar]
- 36.Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Biomacromolecules. 2007;8:1844. doi: 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- 37.del Amo DS, Wang W, Jiang H, Besanceney C, Yan AC, Levy M, Liu Y, Marlow FL, Wu P. J Am Chem Soc. 2010;132:16893. doi: 10.1021/ja106553e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beatty KE, Fisk JD, Smart BP, Lu YY, Szychowski J, Hangauer MJ, Baskin JM, Bertozzi CR, Tirrell DA. ChemBioChem. 2010;11:2092. doi: 10.1002/cbic.201000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laughlin ST, Bertozzi CR. ACS Chem Biol. 2009;4:1068. doi: 10.1021/cb900254y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Proc Natl Acad Sci U S A. 2010;107:10360. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci U S A. 2010;107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeForest CA, Sims EA, Anseth KS. Chem Mater. 2010;22:4783. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeForest CA, Polizzotti BD, Anseth KS. Nat Mater. 2009;8:659. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Prifti F, Song J. Macromolecules. 2011;44:2660. doi: 10.1021/ma200021m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. Chem Rev. 2007;107:5813. doi: 10.1021/cr068415b. [DOI] [PubMed] [Google Scholar]
- 47.Nederberg F, Trang V, Pratt RC, Kim SH, Colson J, Nelson A, Frank CW, Hedrick JL, Dubois P, Mespouille L. Soft Matter. 2010;6:2006. [Google Scholar]
- 48.Nederberg F, Trang V, Pratt RC, Mason AF, Frank CW, Waymouth RM, Hedrick JL. Biomacromolecules. 2007;8:3294. doi: 10.1021/bm700895d. [DOI] [PubMed] [Google Scholar]
- 49.Hiemstra C, Zhong ZY, Dijkstra P, Feijen J. Macromol Symp. 2005;224:119. [Google Scholar]
- 50.Bryant SJ, Anseth KS. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 51.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Song J, Xu J, Filion T, Saiz E, Tomsia AP, Lian JB, Stein GS, Ayers DC, Bertozzi CR. J Biomed Mater Res Part A. 2009;89A:1098. doi: 10.1002/jbm.a.32110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.