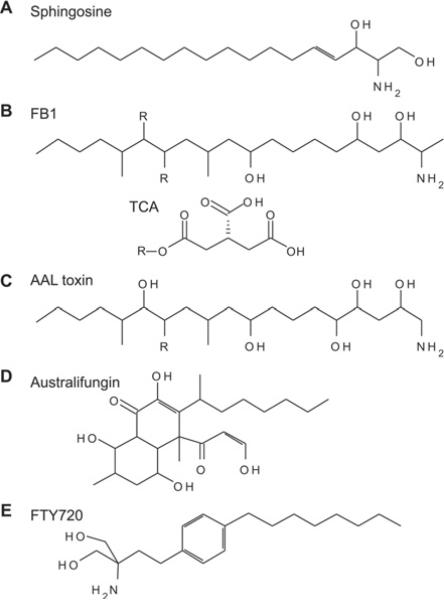
Figure 4. Inhibitors of CerS activity.
Known inhibitors of CerS include sphingosine (A) analogues such as fumonisins (B), a toxin from Alternaria alternata f. sp. lycopersici (AAL toxin) (C) and australifungin (D) [124]. Of these compounds, the FB1 (B) is the best characterized and most widely used to experimentally inhibit CerS. Fumonisins are produced by the fungus Fusarium moniliforme and were found to inhibit CerS activity in vitro and in vivo [125]. The structure of fumonisins consists of a hydrocarbon chain containing side chains including hydroxy and methyl groups and a single amino/amide group. In fumonisin B species, two of these hydroxy groups are esterified to tricarballylic acid (TCA). The TCA groups can be removed from the hydrocarbon backbone via intracellular esterases to form hydrolysed fumonisins (HF) or aminopolyols (AP). Hydrolysed FB1 (HFB1) is less potent than FB1 at inhibiting CerS and cell growth, suggesting that both the backbone and TCA groups are required for efficient binding to these enzymes [126,127]. An interesting characteristic of HFB1 is that it also is a substrate for the CerS reaction [127,128]. The resulting product, N-acyl-HFB1, is considerably more potent in cells than either HFB1 or FB1 (more than 10-fold). However, in vitro, HFB1 is less potent than FB1 [127]. AAL toxin (C) is highly similar in structure to fumonisins, and similarly inhibits CerS activity and elevates sphingoid bases [129,130]. Australifungin (D), a product of the fungus Sporormiella australis, is more potent than FB1 at inhibiting fungal CerS activity [124,131]. However, the β-ketoaldehyde moiety of australifungin can react with the free amines of sphingoid bases causing these to be sequestered, making its experimental use as an in vitro and in vivo CerS inhibitor problematic. FTY720 (fingolimod) (E), a myriocin analogue, has been shown to inhibit CerS through a complex mechanism including both uncompetitive and non-competitive modes [132,133].