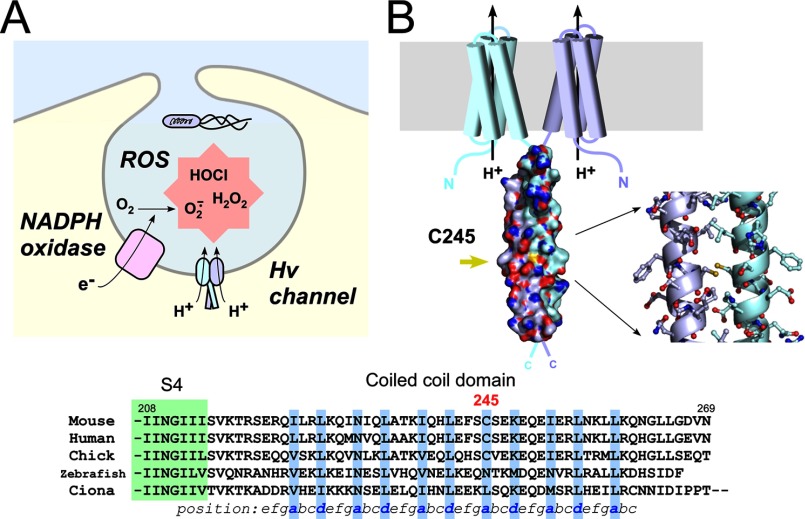
FIGURE 1.
Intersubunit disulfide bonding of Cys-245 in native mVSOP/Hv1. A, schematic drawing of the Hv channel function in the phagosome. The NADPH oxidase produces ROS, and the Hv channel regulates NADPH oxidase activity. H+ efflux through the Hv channel effectively contributes to the charge compensation for electron transfer via NADPH oxidase, which helps to sustain the production of ROS (7). B, structural model of the dimeric unit of mVSOP/Hv1. Shown are the protein surface of the coiled-coil domain and a stick model of Cys-245 and surrounding residues (Protein Data Bank code 3VMX) (13). Sulfur atoms of Cys-245 are colored yellow, and the dual conformation in the model was omitted to facilitate visualization. Also shown is the sequence alignment of the C-terminal cytoplasmic coiled-coil domain of the Hv channel from various species. Coiled-coil residues occupying hydrophobic positions a and d are shown in blue. The green box depicts part of the last transmembrane region (S4).