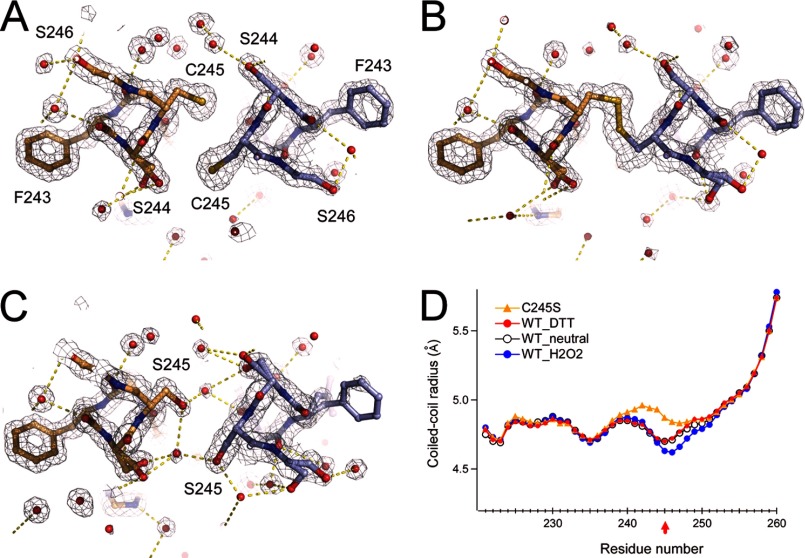
FIGURE 3.
Crystal structure analysis of the disulfide bond in the coiled coil. A and B, structure and 2Fo − Fc maps of Cys-245 and surrounding residues in the presence of 1 mm DTT (A) or 1 mm H2O2 (B). C, structure and 2Fo − Fc maps of the C245S mutant coiled coil. Red spheres depict the oxygen atoms of water molecules. Yellow dashed lines depict polar contacts. Maps are contoured at 1.5σ. Neighboring Ser residues form a dual conformation. D, structural comparison of the WT_neutral (Protein Data Bank code 3VMX), WT_H2O2, WT_DTT, and C245S crystal structures.