FIGURE 1.
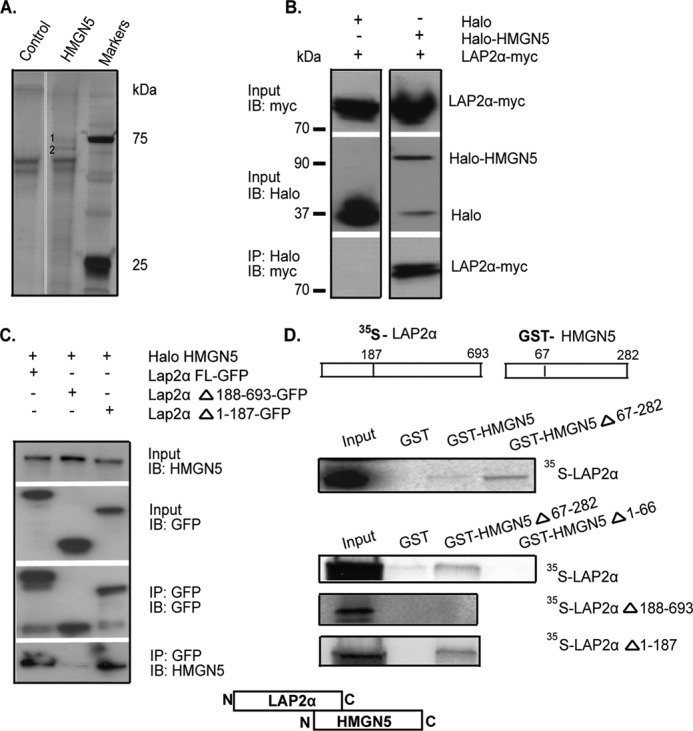
HMGN5 and LAP2α interact in vivo and in vitro. A, silver-stained SDS-PAGE gel of proteins recovered from HaloLink resin. Specific bands are marked as 1 and 2. B, immunoprecipitation of LAP2α-Myc by Halo-HMGN5 from HeLa cell extracts. C, HMGN5 interacts with the C-terminal region of LAP2α. Shown are Western blots (IB) of input and immunoprecipitated proteins from HeLa cells expressing Halo-HMGN5 and LAP2α-GFP constructs. D, GST pulldown assay indicates that the N terminal region of HMGN5 interacts with the C terminus of LAP2α. 35S-labeled LAP2α or its deletion mutants was incubated with purified GST-HMGN5 or its deletion mutants, and the complex was purified on glutathione-Sepharose, fractionated by SDS-PAGE, and visualized by autoradiography. The scheme visualizes that the C-terminal region of LAP2α interacts with the N-terminal region of HMGN5.
