FIGURE 3.
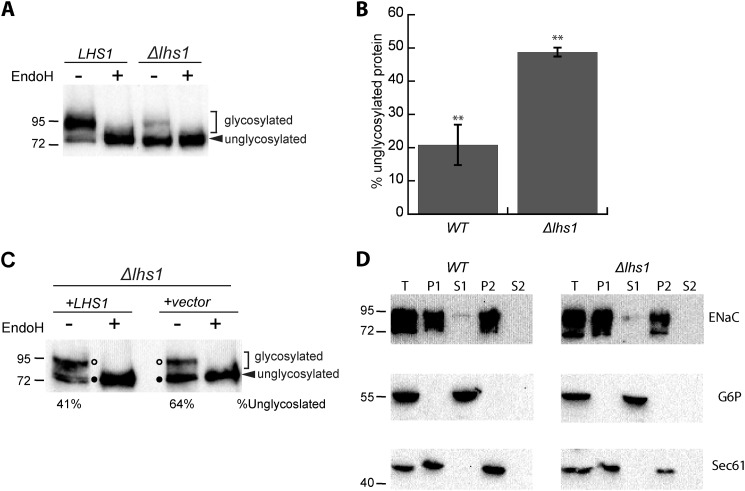
The unglycosylated form of αENaC accumulates in the absence of Lhs1. A, lysates were prepared from LHS1 WT or Δlhs1 yeast expressing a C-terminal HA-tagged form of αENaC and were incubated at 37 °C in the presence or absence of EndoH before performing an immunoblot analysis with anti-HA antiserum to detect ENaC. B, the relative percent of unglycosylated protein was quantitated using the zero time point in Fig. 1A (αENaC). Data represent the means of four experiments ± S.E.; **, p < 0.01 C, lysates were prepared from Δlhs1 yeast expressing a C-terminal, HA-tagged form of αENaC and a plasmid containing Lhs1 or a vector control and were treated with EndoH and analyzed as above. D, lysates were prepared from WT or Δlhs1 yeast expressing a C-terminal HA-tagged form of αENaC and membrane (P1 and P2) and supernatant fractions (S1 and S2) were isolated as described under “Experimental Procedures.” These fractions and an aliquot of the total cell lysate (T) were immunoblotted with anti-HA antisera (ENaC) or anti-G6P as a cytosolic control or anti-Sec61 as an integral ER membrane protein control.