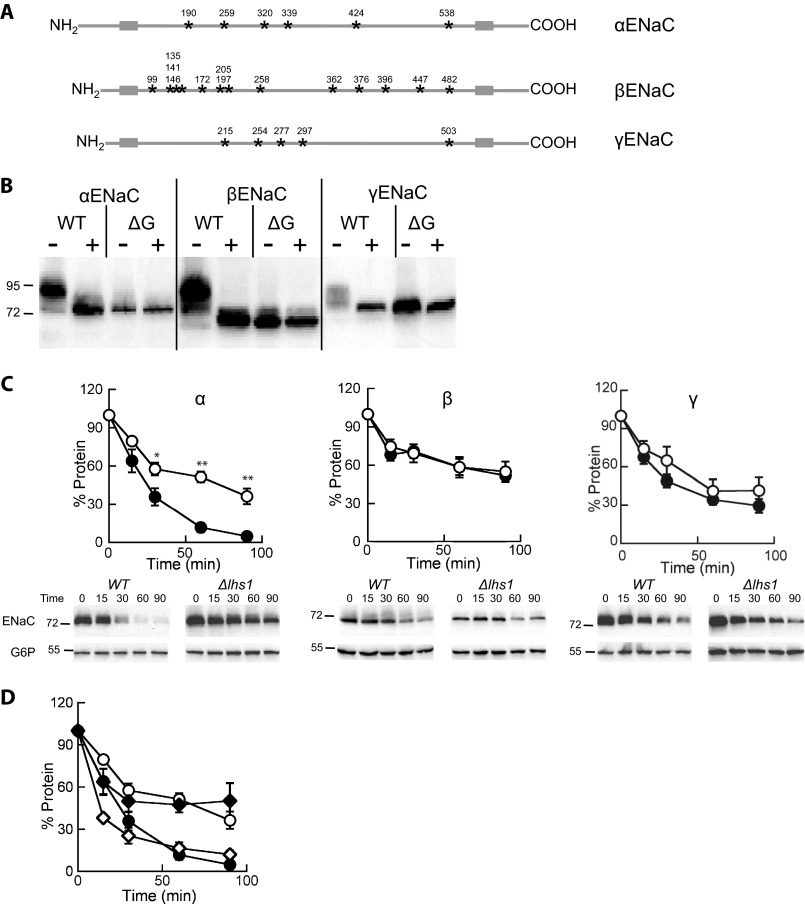
FIGURE 4.
Lhs1 is critical for the degradation of the unglycosylated form of αENaC but is dispensable for the turnover of the unglycosylated versions of β- or γENaC. A, shown is a schematic representation of the ENaC subunits demonstrating the transmembrane segments (gray boxes) and N-linked glycosylation sites (stars) with the amino acid positions noted above each site. B, lysates were prepared from LHS1 WT yeast expressing a C-terminal HA-tagged form of α-, β-, γ-, ΔGα-, ΔGβ-, or ΔGγENaC and were incubated at 37 °C in the presence or absence of EndoH before an immunoblot analysis was performed with anti-HA antiserum to detect ENaC. C, cycloheximide chase reactions were performed as described under “Experimental Procedures” in LHS1 (filled circles) or Δlhs1 (open circles) yeast strains expressing C-terminal HA epitope-tagged ΔGα-, ΔGβ-, or ΔGγENaC. Chase reactions were performed at 37 °C, and lysates were immunoblotted with anti-HA antiserum (ENaC) and with anti-G6P as a loading control. Data represent the means of 7–13 experiments ±S.E.; *, p < 0.05; **, p < 0.001. D, the glycosylated (open diamonds) and unglycosylated (closed diamonds) species from the Δlhs1 αENaC data from Fig. 1A were quantified and compared with the ΔGαENaC data from C. Note that the symbols used in this panel as the same as those used in C.