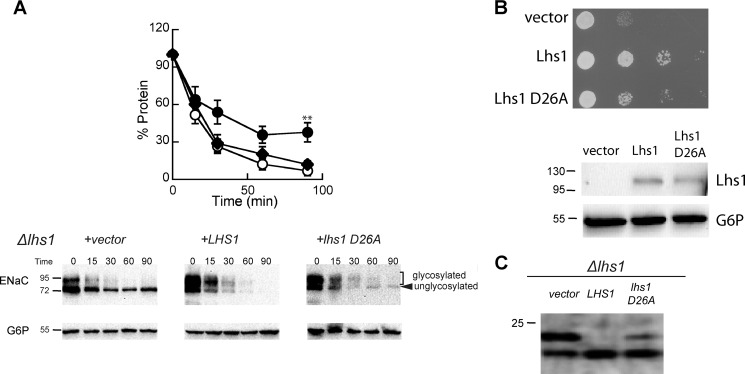
FIGURE 5.
The ATP binding activity of Lhs1 is not required to support the degradation of αENaC. A, cycloheximide chase reactions were performed as described under “Experimental Procedures” in an Δlhs1 yeast strain expressing a C-terminal HA epitope-tagged form of αENaC and either wild-type Lhs1 (open circles), Lhs1 D26A (diamonds), or a vector control (filled circles). Chase reactions were performed at 37 °C, and lysates were immunoblotted with anti-HA antiserum (αENaC) and with anti-G6P as a loading control. Data represent the means of 6–8 experiments ±S.E.; **, p < 0.02, vector versus lhs1 D26A. B, Δlhs1 yeast strains expressing the indicated protein or containing a vector control were serial-diluted and plated on selective medium and incubated at 26 °C. Cells from the plates were resuspended in lysis buffer and lysed, and the total protein was subjected to immunoblot analysis with anti-Lhs1 antisera and anti-G6P as a loading control. C, Δlhs1 yeast strains expressing ΔGppαF-HA and containing a vector engineered for the expression of either wild-type Lhs1 or Lhs1 D26A or a vector control were grown to log phase, and proteins were precipitated as described under “Experimental Procedures” and immunoblotted with anti-HA antisera.