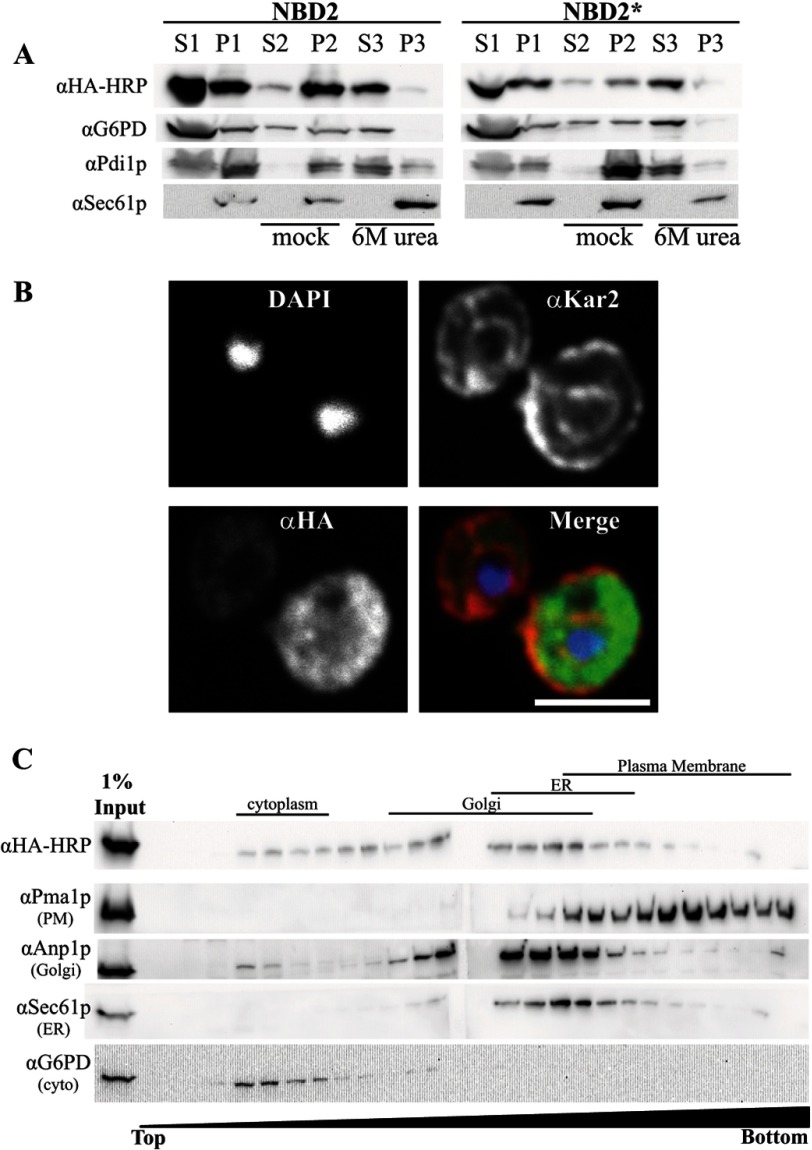
FIGURE 2.
NBD2* is a cytoplasmic membrane-associated protein. A, yeast expressing NBD2 or NBD2* were grown to log phase and disrupted by glass bead lysis (as described under “Experimental Procedures”) to generate a membrane fraction (pellet 1 (P1)) and cytosolic fraction (supernatant 1 (S1)). Equal portions of the pellet fraction were either mock-treated or treated with 6 m urea to remove peripheral proteins (S2/P2 and S3/P3, respectively). Immunoblots were performed with anti-HA-HRP for NBD2, anti-G6PD (cytoplasmic marker), anti-Sec61 (integral membrane marker), and anti-Pdi1p (ER lumenal/peripheral membrane marker). B, NBD2* localization was determined by indirect immunofluorescence confocal microscopy, using mouse anti-HA (NBD2*), rabbit anti-Kar2p (ER lumen), and DAPI to label nuclei. Primary antibodies were decorated with Alexa goat anti-mouse 488 and goat anti-rabbit 568, respectively. Scale bar, ∼5 μm. C, yeast lysates containing NBD2* were analyzed by sucrose gradient centrifugation. Fractions were taken from the top, and the density of sucrose in each fraction increases throughout the gradient. An aliquot of each fraction was analyzed by SDS-PAGE, and after transfer, the nitrocellulose was immunoblotted for Kar2p (ER marker), Anp1 (Golgi marker), Pma1 (plasma membrane marker), and NBD2* (anti-HA-HRP). A lane containing 1% of the total protein loaded on the gradient was also included (1% Input).