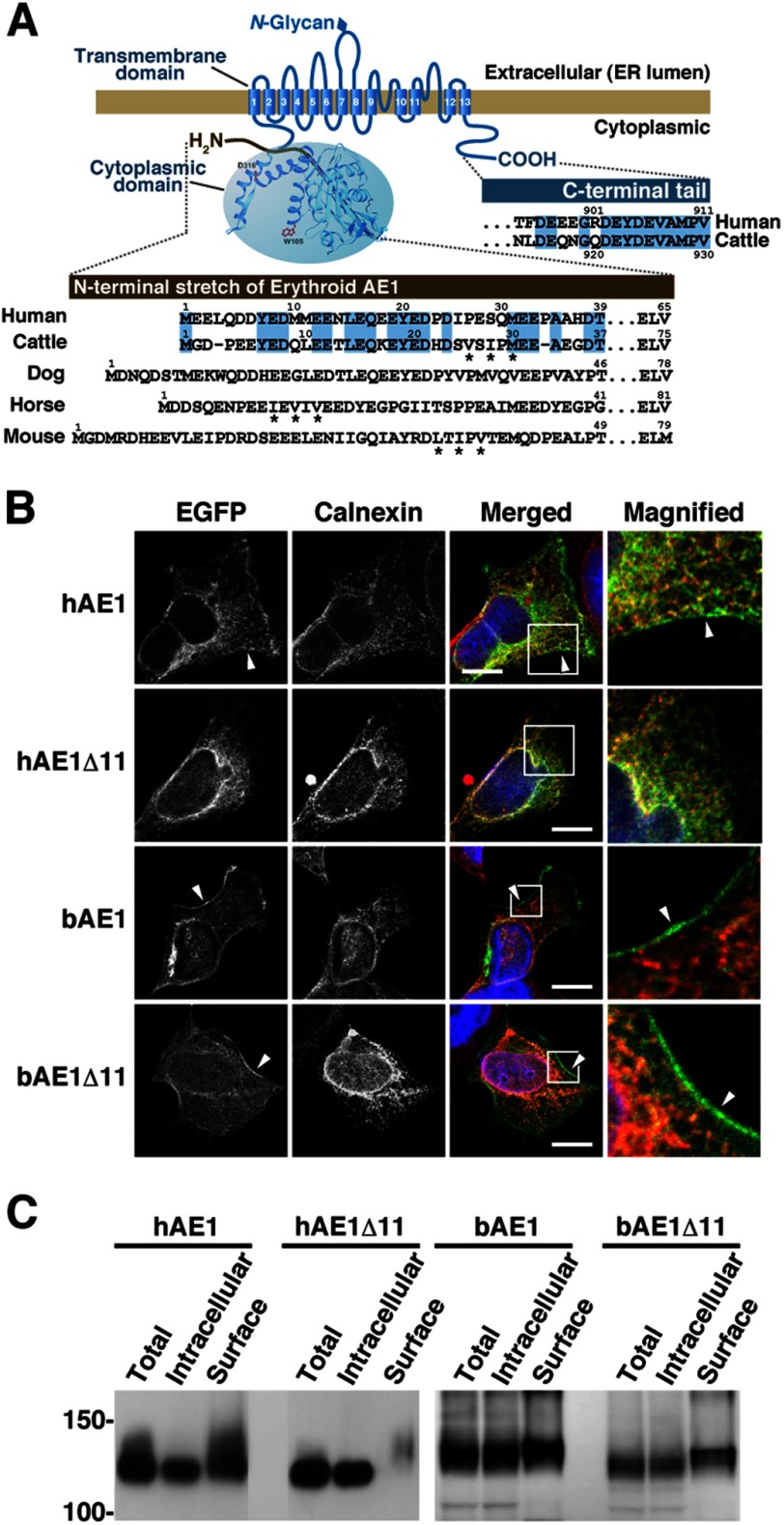
FIGURE 1.
Expression of human and bovine AE1 and their C-terminally truncated mutants in transfected HEK293 cells. A, schematic illustration of erythroid AE1 based on the crystallographic structure of the cytoplasmic domain (36) and the topology model of the transmembrane domain (45). The amino acid sequences of the N- and C-terminal regions of human and bovine AE1 are aligned, and residues highlighted in blue are identical between humans and cattle. The N-terminal sequences of canine, equine, and murine AE1 are also shown. The N-terminal sequence varies between the species. The functions of these amino acid sequences in ER export were analyzed (Figs. 4–7), and asterisks indicate the hydrophobic residues that correspond to the ΦXΦXΦ motif. B, HEK293 cells were transfected with EGFP-tagged hAE1 or bAE1 and their C-terminally truncated mutants. The cells were fixed after 48 h, and EGFP and the ER marker calnexin were visualized. A merged image of EGFP- (green), calnexin- (red), and DAPI-stained nuclei (blue) is shown. The indicated areas in the merged images are magnified, and EGFP fluorescence at the plasma membrane is indicated by arrowheads. Bars, 10 μm. C, at 48 h after transfection, cell surface proteins were labeled with sulfo-NHS-SS-biotin. After solubilization, biotin-labeled proteins were separated from intracellular proteins on NeutrAvidin beads. AE1 proteins in total (Total), intracellular (Intracellular), and cell surface (Surface) fractions were detected by immunoblotting with an anti-GFP antibody. The amount of cell surface fraction loaded is 10-fold higher than that of the other fractions. Migrating positions of size markers are shown in kDa.