Figure 2.
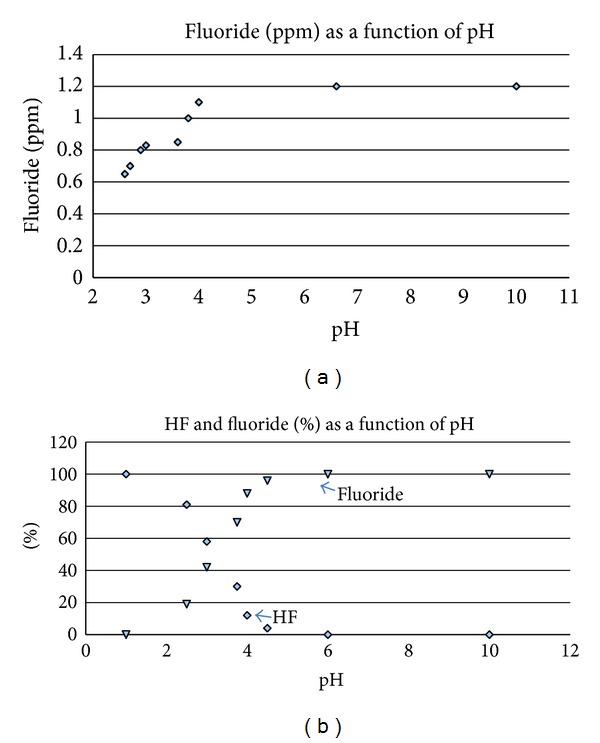
Fluoride protonation depends on prevailing acidity following the equilibrium reaction F− + H+↔HF. (a) All measurements of fluoride ion concentration were made with a LaMotte Instruments fluoride ion specific electrode calibrated with a 1.00 ppm fluoride standard solution in distilled deionized water at room temperature. Readings for the 1.2 ppm true concentration solution progressively decrease as pH decreases. Acidity was adjusted with dilute acetic acid. Stomach acid pH normally varies from 1.5 to 3 (Teitz [18] p. 1072), where fluoride is mostly protonated as hydrofluoric acid HF. (b) The percent contribution to the total fluoride from the free ion F− (triangles) and intact HF (diamonds), calculated theoretically from the Henderson Hasselbach equation pH = pKa + log{[F−]/[HF]} over the pH range 1–10, utilizing the known Ka for HF. HF decreases while F− increases from pH 1 to 5. At pH 3.14 (= pKa = −log 7.2 × 10−4), HF is half dissociated.
