Figure 3.
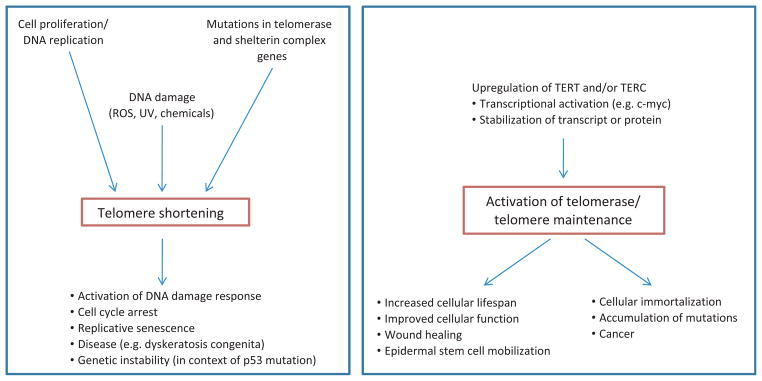
Presented is a summary of factors and activities that lead to either telomere shortening or telomere maintenance, and the possible outcomes in cells, as well as in organisms as a whole. (Left box): Telomere shortening occurs normally during cell division and DNA replication, because of the end replication problem. Mutations in telomerase and shelterin complex genes can lead to accelerated shortening and dysfunctional telomeres. In addition, reactive oxygen species or other DNA-damaging agents cause rapid telomere shortening. The erosion of telomeres to a short critical length activates a DNA damage response, cell cycle arrest and senescence. This causes failure of tissue regeneration and tissue ageing. In the context of p53 mutation, short telomeres can lead to genetic instability, which may play a role in the development of cancer. (Right box): The upregulation of TERT and/or TERC expression through a variety of factors (e.g. transcriptional upregulation, stabilization of the transcript or protein) causes activation of telomerase, which can maintain or even elongate telomeres. In a normal context, this allows stem cell maintenance, cell proliferation and tissue regeneration. Upregulation of TERT has also been shown to be involved in mobilization of epidermal stem cells. Unchecked telomerase activity is associated with cellular immortalization, which is a prerequisite for cancer and can result in accumulation of genetic and epigenetic changes that may increase the likelihood of malignancy. See text for references.
