Abstract
Purpose
The purpose of this study was to determine if carbonic anhydrase inhibitors can restore their efficacy after a period of discontinued use in patients with cystic foveal lesions who demonstrated subsequent worsening in the extent of their foveal cysts after initially exhibiting a favorable response to treatment.
Methods
Retrospective chart review was conducted on all patients with retinitis pigmentosa or X-linked retinoschisis who were either currently on treatment or had been treated with carbonic anhydrase inhibitors for cystic macular lesions. A total of three patients were included in the study.
Results
All three patients exhibited a recurrence of their cystic macular lesions while on treatment with carbonic anhydrase inhibitors. After discontinuing treatment for a period of 1 month to 6 months, all patients showed a favorable response to retreatment as monitored with optical coherence tomography scans.
Conclusion
The present study shows that patients who show signs of recurring macular cysts while still on treatment can have a favorable response when treatment is reinstated after a period of discontinued use of a carbonic anhydrase inhibitor.
The use of carbonic anhydrase inhibitors (CAIs) has been shown to be effective for the improvement of cystoid macular edema (CME) in patients with retinitis pigmentosa (RP),1,2 as well as for the improvement of foveal cystic-appearing lesions in patients with X-linked retinoschisis (XLRS).3,4 It has been our experience that some patients who initially exhibit a favorable response to the drug can show signs of a recurrence of the cystic lesions in the macula while still on treatment.
We analyzed the possibility that discontinuing the drug in these patients for a period of 1 month to 6 months and then reinitiating treatment can once again result in a favorable response. To our knowledge, to date, there has been no similar published study that addresses this important issue.
Patients and Methods
A chart review was conducted on all patients in our clinic with either RP or XLRS who were either currently on treatment or had previously been on treatment with either an oral or topical CAI. A total of 32 patient charts were included in the review. The following patients were excluded: those who had a history of poor medication compliance (n = 2), those who did not show a favorable response initially (n = 5), those who declined further treatment (n = 1), and those who were currently on treatment and had maintained a favorable response since the initiation of treatment (n = 21). We defined a favorable response as a significant improvement in the degree of cystic macular lesions, which was determined by measuring changes in the central foveal thickness and the foveal zone thickness on optical coherence tomography (OCT).
Three patients (2 men, 1 woman) were included in the final study. All the patients had a recurrence of their cystic macular lesions while on treatment. The age range of the patients was 28 years to 67 years with a mean age of 45 years. All three patients were white.
Patient 1 has a diagnosis of Usher syndrome type 2 and is currently being treated with 500 mg of acetazolamide (Diamox; Duramed Pharmaceuticals, Pomona, NY) each day. Patient 2 has a diagnosis of autosomal dominant RP and is currently being treated with 500 mg of acetazolamide every other day, and Patient 3 has a diagnosis of XLRS and is currently on 2% dorzolamide (Trusopt; Merck & Co., Blue Bell, PA) 3 times a day in each eye.
All patients were examined by one of the authors (G.A.F.) to establish the diagnosis of their respective disease. Findings in the patients with RP include night blindness, progressive visual field loss, and either reduced or nondetectable a- and b-wave amplitudes on electroretinogram testing. Additionally, patients exhibit the following clinical characteristics: bone spicule–like retinal pigmentary changes, optic disk pallor, and attenuated retinal vessels.5 Usher syndrome combines a congenital, nonprogressive, neurosensory hearing impairment with the findings of RP.6 X-linked retinoschisis, which affects almost exclusively men, is characterized by a decrease in visual acuity in the first decade of life and the presence of stellate-shaped foveal cystic changes on fundus examination. Approximately 50% of patients will show peripheral retinoschisis. The electroretinogram is characterized by a predominant b-wave reduction.7 None of the patients in our study had additional ocular conditions or were on other medications that could potentially affect visual acuity and/or retinal function.
Before initiating treatment, a baseline OCT scan was obtained on each patient with an OCT-3 commercial instrument (Stratus; Carl-Zeiss Meditec, Dublin, CA). At subsequent follow-up visits, some OCT images for all 3 patients were obtained on an RTVue RT100 system (Optovue, Inc., Fremont, CA). Six OCT images were acquired by 6-mm radial scans on the Stratus system and twelve 6-mm radial scans were acquired using the Optovue system, with both systems centered on fixation. A central foveal thickness was calculated as an average of the measurements obtained. The foveal zone thickness, a region within 1000 μm centered at the foveola, was also measured. A previous study8 has demonstrated a statistically significant difference between the retinal thickness measurements obtained by an RTVue system versus those obtained with the Stratus system. The small differences observed (a mean difference of 8 μm between the 2 systems) were accounted for when analyzing our data on macular thickness measurements and did not alter any of the results or conclusions.
For the patient affected with XLRS, a foveal thickness change of >19.6% (mean ± 2 SD) and a foveal zone thickness change from pretreatment of >17.1% (mean ± 2 SD) were used as statistically significant intervisit changes.3 For those patients affected with RP, a change of more than 16% foveal thickness and more than 11% in foveal zone thickness were considered significant.9 We compared all subsequent retinal thickness measurements with those obtained at the baseline visit.
After initial OCT measurements were obtained, Patient 1 was assigned to 500 mg of acetazolamide daily and Patient 2 was initially assigned to dorzolamide 3 times a day in each eye, which resulted in an unsatisfactory response and was therefore switched to 500 mg acetazolamide every other day. Patient 3 was assigned to dorzolamide 3 times a day in each eye. Patient 1 returned in 1 month and both Patients 2 and 3 returned in 2 months for their first follow-up visit that consisted of repeat OCT measurements. After the first follow-up visit, each patient was seen several times (follow-up visits ranged from 2 to 6 months from the previous visit) to monitor the response to treatment. Patient 1 had a total of 27 visits over a period of 71 months, Patient 2 had 14 visits over a period of 61 months, and Patient 3 had 16 visits over a period of 73 months. At each visit, a repeat OCT measurement was obtained.
This study was approved by the institutional review board of the University of Illinois at Chicago. As per Health Insurance Portability and Accountability Act’s regulations, all patients included in the study signed an informed consent form.
Results
Patient 1
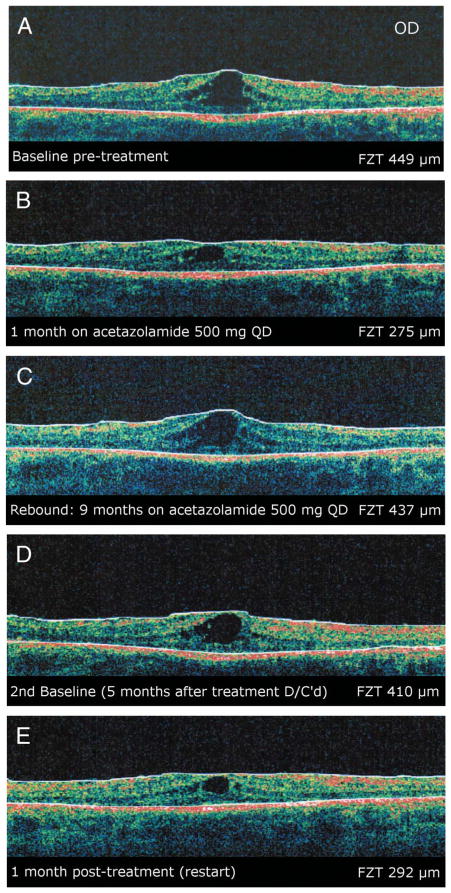
A twenty-eight-year-old man with the diagnosis of Usher syndrome type 2 responded to 500 mg of acetazolamide with improvement of his CME for a period of 9 months before exhibiting a recurrence in the degree of CME. At this point, the drug was discontinued for a period of 2 months after which he was placed on dorzolamide for 1 month, which yielded no change to the extent of his CME. The dorzolamide was discontinued, and 2 months later, the patient was restarted on 500 mg of acetazolamide. After a period of 1 month, he demonstrated a significant response with improvement in his macular cysts as seen on OCT measurements. Over the course of the next 4 years, the patient rebounded 3 more times and after each rebound was taken off acetazolamide for either 2 or 3 months and then retreated, whereby he exhibited a favorable response to the retreatment (Figure 1).
Fig. 1.
Patient 1. A, Baseline OCT image (90°) of the right eye showing CME. B, Optical coherence tomography image of the same eye after 1 month of treatment with acetazolamide. C, Optical coherence tomography image of the same eye 9 months later showing a rebound of macular cysts from baseline. Acetazolamide was discontinued at this time. D, Optical coherence tomography image of the same eye 5 months after discontinuing treatment. Treatment with acetazolamide was restarted at this time. E, Optical coherence tomography image of the same eye 1 month post retreatment demonstrating a reduction in size of the macular cysts. All images were obtained on a Stratus system.
Patient 2
A sixty-seven-year-old woman, diagnosed with autosomal dominant RP, was initially treated with dorzolamide for CME to which she did not have a significant response. She was then assigned to 500 mg of acetazolamide every other day to which she showed a significant response with a decrease in her CME on OCT scans. Four months later, she displayed a recurrence of the cystic changes while on treatment and the drug was discontinued for 5 months after which the use of acetazolamide every other day was restarted. She again demonstrated improvement in the CME, which has been maintained to date.
Patient 3
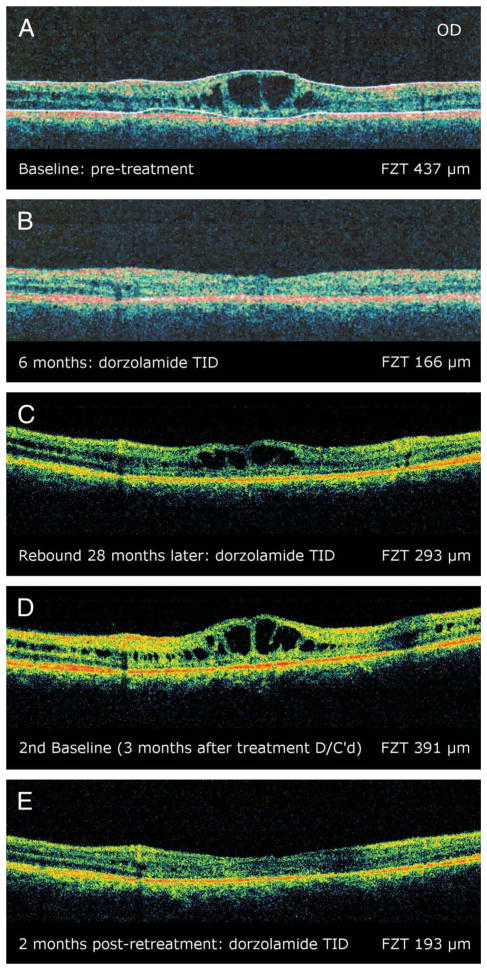
A forty-year-old man with the diagnoses of XLRS began treatment with dorzolamide three times daily in each eye to which he had a favorable response with a reduction of his foveal cysts on repeat OCT scans. Two years and 4 months later, the patient experienced a rebound of the foveal cystlike lesions, and the drops were discontinued for 3 months. On retreatment, an improvement in his cystic foveal lesions was observed which has been maintained to date (Figure 2).
Fig. 2.
Patient 3. A, Baseline OCT image (90°) of the right eye showing foveal cystic-appearing lesions. B, Optical coherence tomography image of the same eye after 6 months of treatment on dorzolamide. C, Optical coherence tomography image of the same eye 2 years and 4 months later, illustrating a recurrence of the foveal cysts while on treatment. Treatment was discontinued at this time. D, Optical coherence tomography of the same eye 3 months after discontinuing treatment. Treatment was reinstated at this time. E, Optical coherence tomography of the same eye 2 months post retreatment showing an improvement in the cystic lesions. The first two images were obtained on a Stratus system, whereas the last three images were obtained on an Optovue system.
All three patients demonstrated a one-line visual acuity variation measured with a Snellen projection chart, from baseline to most recent response to retreatment. These results were not considered significant when compared with the criterion set by a previous study10 where an increase of greater than or equal to seven letters was considered a significant change.
Discussion
Carbonic anhydrase is one of the most ubiquitous enzyme systems in the body. In the retina, carbonic anyhdrase II is found in the red/green cones and within the Mueller cells. The retinal pigment epithelium (RPE) contains primarily the membrane-bound form of the enzyme, which controls and adjusts the extracellular pH gradients produced by the metabolic activity of cells. It may also act as a bicarbonate channel at this level.11
The clinical effect of CAIs has been demonstrated through the modulation of membrane-bound carbonic anhydrase IV receptors present in the retinal RPE layer.4 The administration of CAIs has been shown to enhance the fluid transporter present in the RPE barrier, as well as enhance retinal adhesiveness, and is therefore thought that the response of CME to CAIs is better in those patients with diffuse RPE disease.11
In certain patients, the resolving cystic spaces may result in an improvement in visual acuity.1,2 Although neither a clinically nor statistically significant difference in improvement of visual acuity was observed in our patients, it is possible that maintaining retinal anatomy in a more normal state may delay further visual acuity loss because of chronic macular edema.
We observed that some patients may show signs of recurrence of their macular cysts while still on treatment. We now established that in these patients, discontinuing the drug for a period of 1 month to 6 months and then restarting the treatment can reinstate a favorable response to treatment in at least some of these patients. We hypothesize that while a patient is on the drug, the pumping mechanism of the RPE is under additional stress eventually compromising its function to transport fluid to the choroid, allowing for an eventual rebound of macular cystic changes to occur in some patients. Removing the drug might allow the metabolic pump to partially recover and therefore facilitate the ability for a future response to treatment. Based on this working notion, we are treating more of our patients on an alternate day basis with the intention of potentially preventing or prolonging the development of a rebound effect.
Acknowledgments
Supported in part by The Foundation Fighting Blindness, Owing Mills, MD, and Grant Healthcare Foundation, Chicago, IL (G.A.F.); National Institutes of Health core grant EY01792; and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article.
References
- 1.Fishman GA, Gilbert LD, Fiscella RG, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol. 1989;107:1445–1452. doi: 10.1001/archopht.1989.01070020519031. [DOI] [PubMed] [Google Scholar]
- 2.Grover S, Fishman GA, Fiscella RG, Adeleman AE. Efficacy of dorzolamide hydrochloride in the management of chronic cystoid edema in patients with retinitis pigmentosa. Retina. 1997;17:222–231. doi: 10.1097/00006982-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina. 2006;26:741–745. doi: 10.1097/01.iae.0000237081.80600.51. [DOI] [PubMed] [Google Scholar]
- 4.Walia S, Fishman GA, Molday RS, et al. Relation of response to treatment with dorzolamide in X-linked retinoschisis to the mechanism of functional loss in retinoschisin. Am J Ophthalmol. 2009;147:111–115. doi: 10.1016/j.ajo.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman GA, Kumar A, Jospeh ME, Torok N, Anderson RJ. Usher’s syndrome. Arch Ophthalmol. 1983;101:1367–1764. doi: 10.1001/archopht.1983.01040020369005. [DOI] [PubMed] [Google Scholar]
- 6.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 7.George ND, Yates JR, Moore AT. Clinical features in affected males with X-linked retinoschisis. Arch Ophthalmol. 1996;114:274–280. doi: 10.1001/archopht.1996.01100130270007. [DOI] [PubMed] [Google Scholar]
- 8.Menke MN, Dabov S, Sturm V. Comparison of three different optical coherence tomography models for total macular thickness measurements in health controls. Ophthalmologica. 2009;223:352–356. doi: 10.1159/000226600. [DOI] [PubMed] [Google Scholar]
- 9.Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141:850–858. doi: 10.1016/j.ajo.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Grover S, Fishman GA, Gilbert LD, Anderson RJ. Reproducibility of visual acuity measurements in patients with retinitis pigmentosa. Retina. 1997;17:33–37. doi: 10.1097/00006982-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97:387–397. doi: 10.1023/a:1002143802926. [DOI] [PubMed] [Google Scholar]