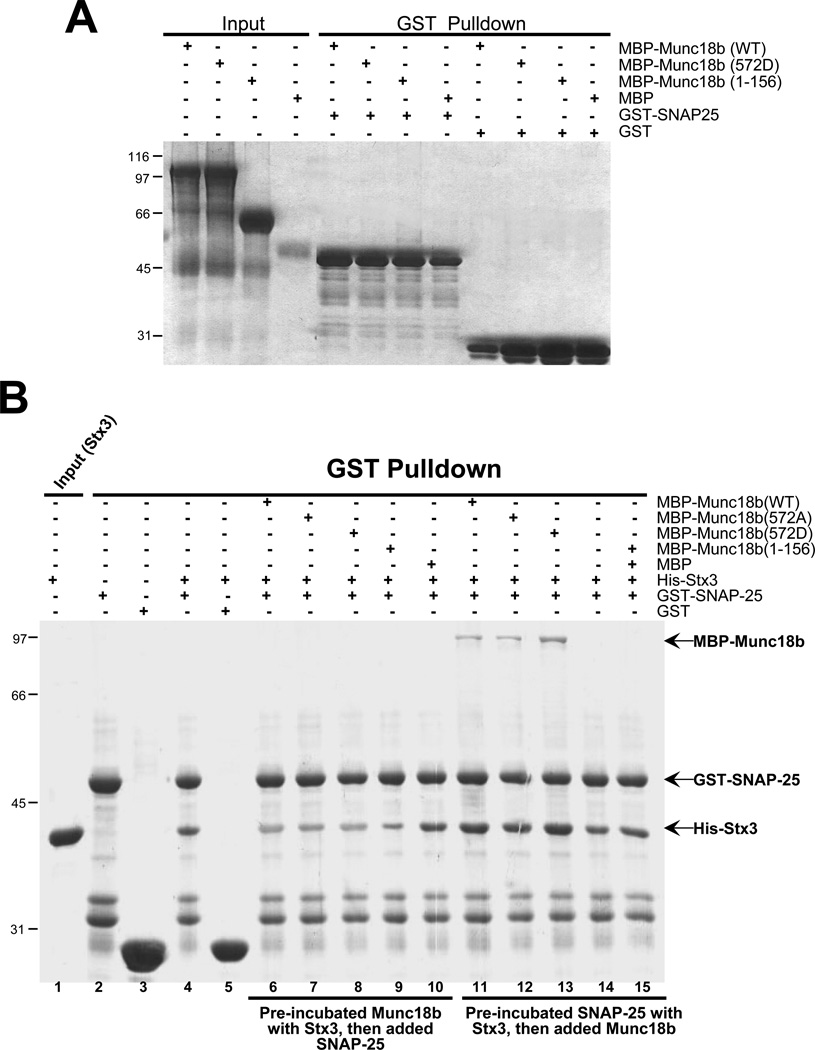
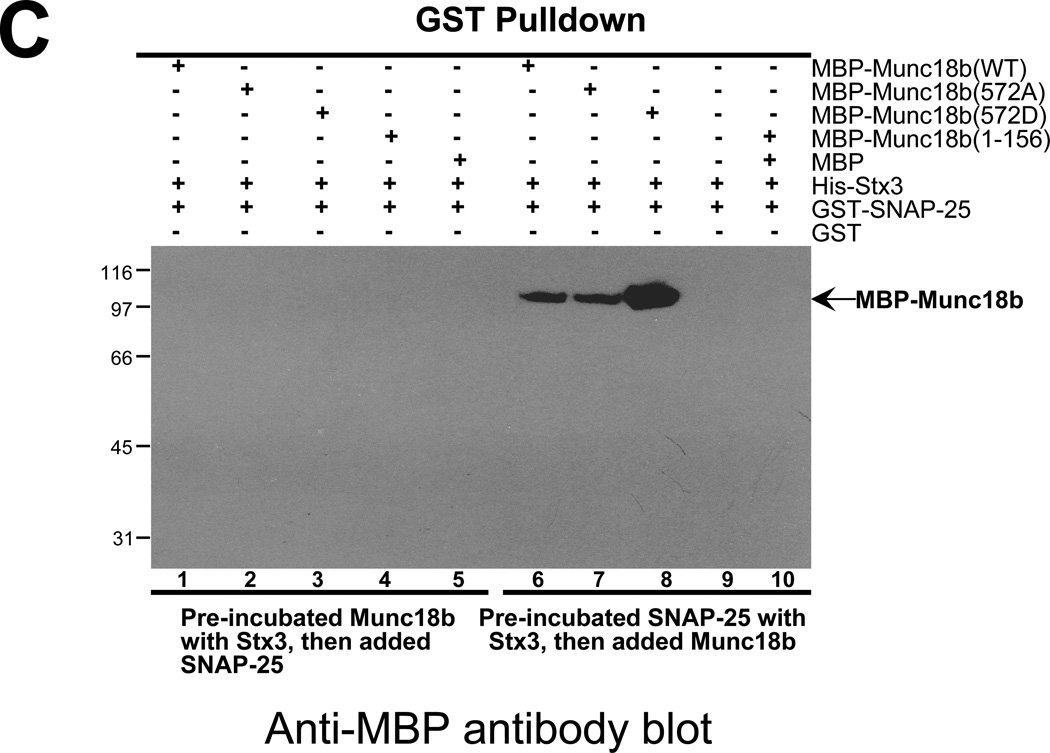
Fig. 3. CDK5 phoshorylation enhances the association of Munc18b with Stx3-SNAP25 complex in vitro.
A. Munc18b bears no SNAP25-binding activity. GST-tagged SNAP25 on glutathione-agarose beads was loaded with purified MBP-tagged full-length and various deletion proteins of Munc18b. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that neither Munc18b1–156 nor Munc18b540–593 binds SNAP25.
B. CDK5 phosphorylation promotes the binding of Munc18b to the Stx3-SNAP25 complex. GST-tagged SNAP25 on glutathione-agarose beads was either incubated with Stx3 first prior to the loading of purified Munc18b, or incubated with a mixture of Munc18b proteins with Stx3. Bound proteins were fractionated by SDS-PAGE and stained with Coomassie Blue. Note that full-length Munc18b but not Munc18b1–156 binds only to pre-formed Stx3-SNAP25 complex while CDK5-mediated phosphorylation promotes the association of Munc18b to Stx3-SNAP25 complex (lane 13 vs lane 12).
C. Phospho-mimicking Munc18b binds better to the Stx3-SNAP25 complex. Western blotting analysis with MBP antibody demonstrates that CDK5-mediated phosphorylation of T572 promotes the association of full-length Munc18b (lane 8 vs lane 7) but not Munc18b1–156 with performed Stx3-SNAP25 complex in vitro.