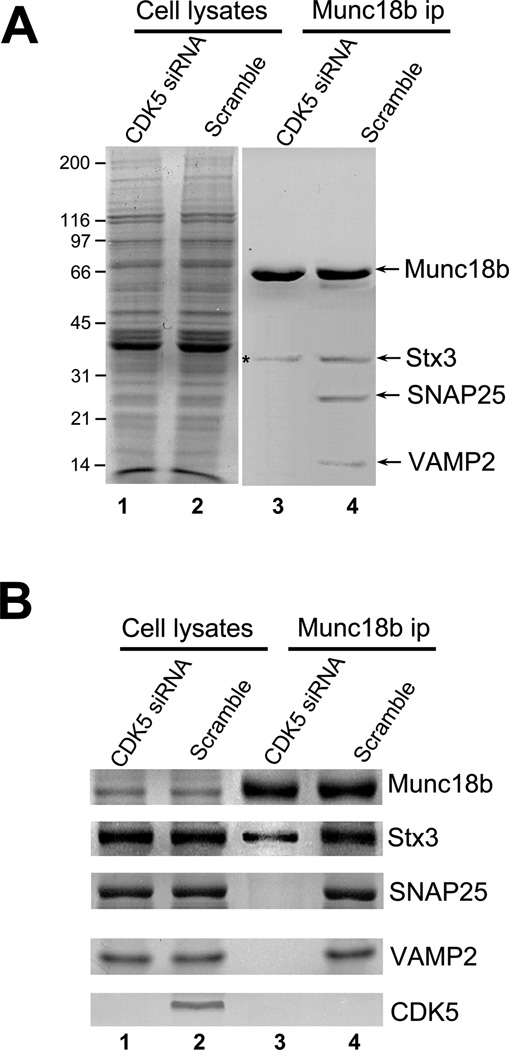
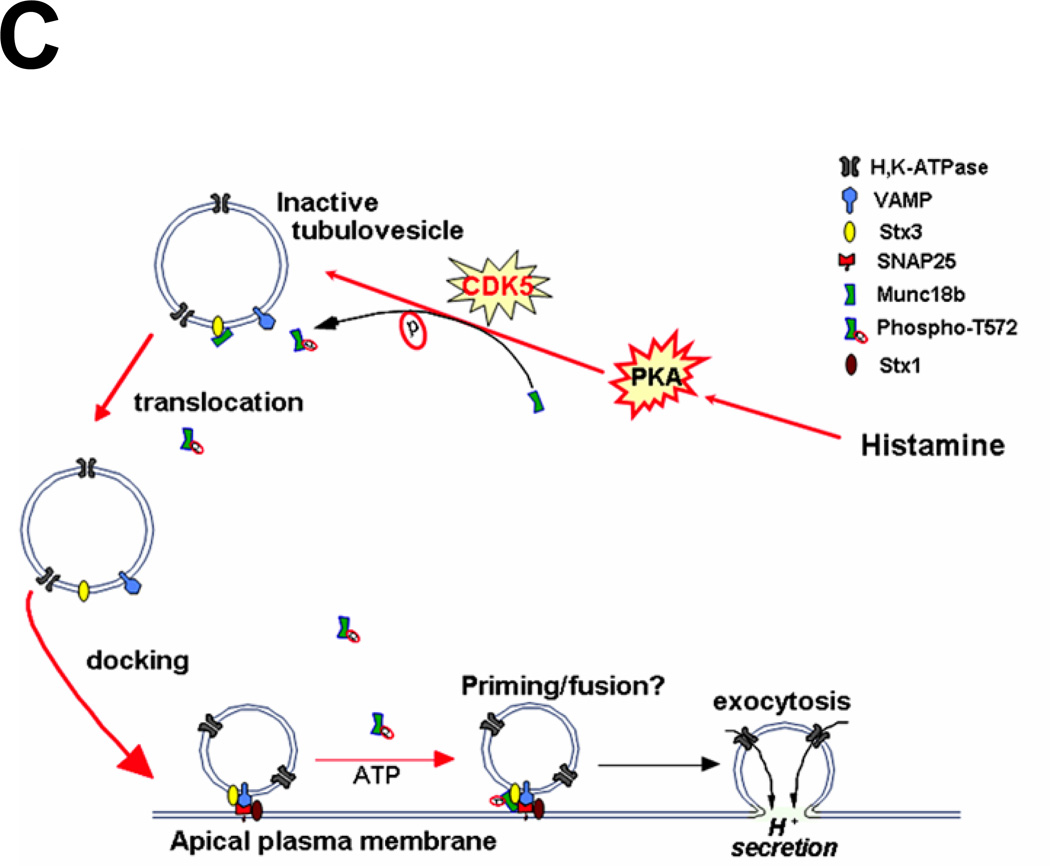
Fig. 4. CDK5-mediated phosphorylation of Munc18 is essential for SNARE complex formation in vivo.
A. CDK5 is essential for formation of a stable Munc18b-SNARE complex in secreting parietal cells. Aliquots of cell lysates from histamine-treated cultured parietal cells, pre-transfected with CDK5 siRNA or scramble oligonucleotides, were incubated with CNBr-coupled Munc18b antibody beads. The beads were washed and bound proteins were fractionated by SDS-PAGE and stained with silver. Note that Munc18b antibody beads pull down several polypeptides corresponding to Stx3, SNAP25 and VAMP2 from control siRNA-treated cells.
B. Repression of CDK5 prevents the formation of a stable Munc18b-SNARE complex in parietal cells. Western blotting analyses with various antibodies to SNAREs confirm that CDK5 is essential for the association of Munc18b with performed Stx3-SNAP25-VAMP complex in vivo.
C. Schematic diagram to propose functional role of CDK5-mediated phosphorylation of Munc18b in parietal cell secretion stimulated by histamine.